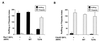ARF6 is required for growth factor- and rac-mediated membrane ruffling in macrophages at a stage distal to rac membrane targeting - PubMed (original) (raw)
ARF6 is required for growth factor- and rac-mediated membrane ruffling in macrophages at a stage distal to rac membrane targeting
Q Zhang et al. Mol Cell Biol. 1999 Dec.
Abstract
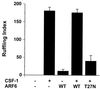
Activation of Rac1, a member of the Rho family of GTPases, is associated with multiple cellular responses, including membrane ruffling and focal complex formation. The mechanisms by which Rac1 is coupled to these functional responses are not well understood. It was recently shown that ARF6, a GTPase implicated in cytoskeletal alterations and a membrane recycling pathway, is required for Rac1-dependent phagocytosis in macrophages (Q. Zhang et al., J. Biol. Chem. 273:19977-19981, 1998). To determine whether ARF6 is required for Rac1-dependent cytoskeletal responses in macrophages, we expressed wild-type (WT) or guanine nucleotide binding-deficient alleles (T27N) of ARF6 in macrophages coexpressing activated alleles of Rac1 (Q61L) or Cdc42 (Q61L) or stimulated with colony-stimulating factor 1 (CSF-1). Expression of ARF6 T27N but not ARF6 WT inhibited ruffles mediated by Rac1 Q61L or CSF-1. In contrast, expression of ARF6 T27N did not inhibit Rac1 Q61L-mediated focal complex formation and did not impair Cdc42 Q61L-mediated filopodial formation. Cryoimmunogold electron microscopy demonstrated the presence of ARF6 in membrane ruffles induced by either CSF-1 or Rac1 Q61L. Addition of CSF-1 to macrophages led to the redistribution of ARF6 from the interior of the cell to the plasma membrane, suggesting that this growth factor triggers ARF6 activation. Direct targeting of Rac1 to the plasma membrane did not bypass the blockade in ruffling induced by ARF6 T27N, indicating that ARF6 regulates a pathway leading to membrane ruffling that occurs after the activation and membrane association of Rac. These data demonstrate that intact ARF6 function is required for coupling activated Rac to one of several effector pathways and suggest that a principal function of ARF6 is to coordinate Rac activation with plasma membrane-based protrusive events.
Figures
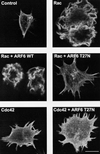
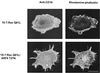
FIG. 1
Effect of ARF6 T27N on Rac1- and Cdc42-mediated cytoskeletal alterations in macrophages. Adherent RAW LacR/FMLPR.2 cells transfected with Myc-Rac1 Q61L (Rac), Myc-Cdc42 Q61L (Cdc42), ARF6 WT, or ARF6 T27N were fixed and stained with a MAb against Myc (to detect either Rac or Cdc42), rabbit antiserum against ARF6, and rhodamine-phalloidin (to detect F-actin). Confocal fluorescence micrographs showing rhodamine-phalloidin fluorescence micrographs of representative cells are shown. Bar = 10 μm.
FIG. 2
Quantitation of membrane ruffling or filopodia formation of Rac1 Q61L (A)- or Cdc42 61L (B)-expressing macrophages also expressing the indicated ARF6 alleles. Data represent the means ± SEM (n = 4).
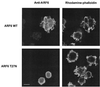
FIG. 3
Effect of ARF6 T27N on focal complex formation induced by Rac1 Q61L in macrophages. Adherent RAW LacR/FMLPR.2 cells transfected with the indicated constructs were fixed and stained with a MAb against Myc (to detect Myc-Rac1 Q61L), rabbit antiserum against ARF6 (to detect cells expressing ARF6 T27N), and MAb PY-99 (to detect phosphotyrosine). Representative confocal micrographs showing Myc (left panels) and PY-99 staining (right panels) are shown. Note prominent phosphotyrosine-rich focal complexes in cells expressing Myc-Rac1 Q61L but not in surrounding nonexpressors. Bar = 10 μm.
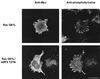
FIG. 4
Effect of ARF T27N on CSF-1-induced membrane ruffling in macrophages. Adherent RAW LacR/FMLPR.2 cells transfected with the indicated constructs were incubated in the presence or absence of 10 ng of CSF-1 per ml for 5 min at 37°C, fixed, and stained with anti-ARF6 and rhodamine-phalloidin. Representative confocal fluorescence micrographs are shown. Bar = 10 μm.
FIG. 5
Quantitation of membrane ruffling induced by CSF-1 in macrophages expressing the indicated constructs in nonexpressing controls. Data represent the means ± SEM (n = 5).
FIG. 6
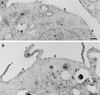
Effect of CSF-1 on the localization of ARF6 in macrophages. Adherent RAW LacR/FMLPR.2 cells transfected with HA-ARF6 were incubated in the absence (A) or presence (B) of 10 ng of CSF-1 for 5 min at 37°C. Ultrathin cryosections were labeled with monoclonal anti-HA followed by rabbit anti-mouse IgG and anti-rabbit IgG–10-nm-diameter gold. ARF6 localized to the plasma membrane (p) and intracellular vesicles and scattered throughout the cytosol. There is also some labeling in the mitochondria (m). Membrane ruffles (r) are apparent in cells incubated with CSF-1 (B). Note that the density of ARF6 labeling is the same for the ruffles and the adjacent areas of the plasma membrane. n, nucleus. Bars = 500 nm.
FIG. 7
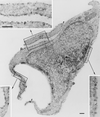
Localization of ARF6 and Rac1 in cells coexpressing Myc-Rac1 Q61L and HA-ARF6 WT. Cryosections of adherent RAW LacR/FMLPR.2 cells transfected with the indicated constructs were double labeled with mouse anti-HA followed by rabbit anti-mouse IgG and anti-rabbit IgG–5-nm-diameter gold and with mouse anti-Myc followed by anti-mouse IgG–10-nm-diameter gold. Labeling with Rac1 (10-nm-diameter gold) can be seen on the plasma membrane (p) and on the membrane of the ruffles (r). The insets show a higher magnification of the marked areas showing the labeling with ARF6 (5-nm-diameter gold) and Rac1 (10-nm-diameter gold). The intensity of both labels on the membrane ruffles and on adjacent nonruffling areas of the plasma membrane is equivalent. Bars = 300 nm.
FIG. 8
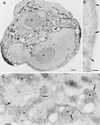
Localization of HA-ARF6 T27N in cells coexpressing Myc-Rac1 Q61L. Cryosections of adherent RAW LacR/FMLPR.2 cells transfected with the indicated constructs were double labeled with a mixture of rabbit anti-HA and mouse anti-Myc followed by a mixture of goat anti-rabbit IgG–10-nm-diameter gold and goat anti-mouse IgG–5-nm-diameter gold. (A) Low magnification of a cell expressing both constructs. n, nucleus. (B) Higher magnification of marked area in panel A showing the plasma membrane labeled only with Rac1 (5-nm-diameter gold) (arrows). (C) Higher magnification of the marked area in panel A showing endocytic vesicles (e) labeled for both ARF6 (10-nm-diameter gold) (large arrows) and Rac1 (5-nm-diameter gold) (small arrows). m, mitochondria. Bars = 1,000 nm (A), 100 nm (B), and 200 nm (C).
FIG. 9
Effect of ARF6 T27N on membrane ruffles induced by expression of a plasma membrane-targeted fusion protein containing Rac1 Q61L in macrophages. RAW LacR/FMLPR.2 cells were transfected with either 16:7:Myc-Rac1 Q61L or 16:7:Myc-Rac1 Q61L and ARF6 T27N. Expression of 16:7:Myc-Rac1 Q61L at the plasma membrane was confirmed by cell surface staining of CD16 (left panels); F-actin was visualized following subsequent permeabilization and staining with rhodamine-phalloidin (right panels). Bar = 10 μm.
Similar articles
- Arf6 recruits the Rac GEF Kalirin to the plasma membrane facilitating Rac activation.
Koo TH, Eipper BA, Donaldson JG. Koo TH, et al. BMC Cell Biol. 2007 Jul 18;8:29. doi: 10.1186/1471-2121-8-29. BMC Cell Biol. 2007. PMID: 17640372 Free PMC article. - ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements.
Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. Radhakrishna H, et al. J Cell Sci. 1999 Mar;112 ( Pt 6):855-66. doi: 10.1242/jcs.112.6.855. J Cell Sci. 1999. PMID: 10036235 - Arf nucleotide binding site opener [ARNO] promotes sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 β-cells and rat islets.
Jayaram B, Syed I, Kyathanahalli CN, Rhodes CJ, Kowluru A. Jayaram B, et al. Biochem Pharmacol. 2011 Apr 15;81(8):1016-27. doi: 10.1016/j.bcp.2011.01.006. Epub 2011 Jan 26. Biochem Pharmacol. 2011. PMID: 21276423 Free PMC article. - Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.
Lim L, Manser E, Leung T, Hall C. Lim L, et al. Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review. - The role of Rho GTPases' substrates Rac and Cdc42 in osteoclastogenesis and relevant natural medicinal products study.
Liu Y, Dou Y, Yan L, Yang X, He B, Kong L, Smith W. Liu Y, et al. Biosci Rep. 2020 Jul 31;40(7):BSR20200407. doi: 10.1042/BSR20200407. Biosci Rep. 2020. PMID: 32578854 Free PMC article. Review.
Cited by
- Arf6 recruits the Rac GEF Kalirin to the plasma membrane facilitating Rac activation.
Koo TH, Eipper BA, Donaldson JG. Koo TH, et al. BMC Cell Biol. 2007 Jul 18;8:29. doi: 10.1186/1471-2121-8-29. BMC Cell Biol. 2007. PMID: 17640372 Free PMC article. - Characterization of the Rac-GAP (Rac-GTPase-activating protein) activity of beta2-chimaerin, a 'non-protein kinase C' phorbol ester receptor.
Caloca MJ, Wang H, Kazanietz MG. Caloca MJ, et al. Biochem J. 2003 Oct 15;375(Pt 2):313-21. doi: 10.1042/BJ20030727. Biochem J. 2003. PMID: 12877655 Free PMC article. - The cytohesin coiled-coil domain interacts with threonine 276 to control membrane association.
Hiester KG, Santy LC. Hiester KG, et al. PLoS One. 2013 Nov 26;8(11):e82084. doi: 10.1371/journal.pone.0082084. eCollection 2013. PLoS One. 2013. PMID: 24303080 Free PMC article. - ADP-ribosylation factor as a novel target for corneal neovascularization regression.
Dai C, Liu G, Li L, Xiao Y, Zhang X, Lu P. Dai C, et al. Mol Vis. 2012;18:2947-53. Epub 2012 Dec 12. Mol Vis. 2012. PMID: 23288987 Free PMC article. - Endogenous ARF6 interacts with Rac1 upon angiotensin II stimulation to regulate membrane ruffling and cell migration.
Cotton M, Boulay PL, Houndolo T, Vitale N, Pitcher JA, Claing A. Cotton M, et al. Mol Biol Cell. 2007 Feb;18(2):501-11. doi: 10.1091/mbc.e06-06-0567. Epub 2006 Nov 22. Mol Biol Cell. 2007. PMID: 17122362 Free PMC article.
References
- Allen W E, Jones G E, Pollard J W, Ridley A J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. - PubMed
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
- Anand-Apte B, Zetter B R, Viswanathan A, Qiu R G, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;272:30688–30692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous