The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1 - PubMed (original) (raw)
The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1
C L Bevan et al. Mol Cell Biol. 1999 Dec.
Abstract
The androgen receptor is unusual among nuclear receptors in that most, if not all, of its activity is mediated via the constitutive activation function in the N terminus. Here we demonstrate that p160 coactivators such as SRC1 (steroid receptor coactivator 1) interact directly with the N terminus in a ligand-independent manner via a conserved glutamine-rich region between residues 1053 and 1123. Although SRC1 is capable of interacting with the ligand-binding domain by means of LXXLL motifs, this interaction is not essential since an SRC1 mutant with no functional LXXLL motifs retains its ability to potentiate androgen receptor activity. In contrast, mutants lacking the glutamine-rich region are inactive, indicating that this region is both necessary and sufficient for recruitment of SRC1 to the androgen receptor. This recruitment is in direct contrast to the recruitment of SRC1 to the estrogen receptor, which requires interaction with the ligand-binding domain.
Figures
FIG. 1
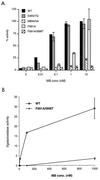
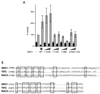
The AR contains AF2, which is essential for function. (A) HeLa cells were plated in 24-well plates and transfected as described in Materials and Methods. Transfected cells were treated with various concentrations (conc) of the synthetic androgen mibolerone (MB) for 18 to 24 h. CAT activity was assayed and corrected for β-galactosidase activity. The activity of wild-type AR (WT) in the presence of 10 nM mibolerone was set at 100% for each experiment, and other values are expressed relative to this. (B) The LBD of the AR contains a ligand-dependent activation function. The LBD fused to a heterologous DBD was expressed in yeast strain W303-1B along with a lacZ reporter vector. Individual transformants were incubated in liquid culture overnight with various concentrations of mibolerone, and cells were pelleted and assayed for β-galactosidase activity and protein content as described in Materials and Methods. The experiment was repeated with five individual transformants for the wild type and three transformants for the mutant construct.
FIG. 2
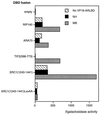
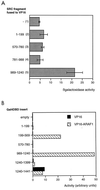
The AR interacts with coactivator proteins via the AF2 core. A two-hybrid assay was performed with yeast and coactivator fragments or full-length protein, as indicated, as bait. The activity of the lacZ reporter in the presence of the VP16 activation domain alone (hatched bars) or fused to the AR LBD without (black bars) or with (gray bars) androgen was measured, and values represent intrinsic activities of the coactivator and the ligand-dependent interaction with AR. NH, no hormone; MB, mibolerone.
FIG. 3
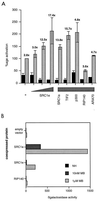
Coactivators potentiate the activities of the full-length AR and AF2 alone. (A) COS-1 cells were transfected as described in Materials and Methods. The coactivator was added at 200 ng per well, or various concentrations (20, 50, and 200 ng) of SRC1e were added. After transfection, cells were incubated with or without 10 nM mibolerone for 18 to 24 h before being harvested and assayed for CAT and β-galactosidase activities. Black bars show activity in the absence and gray bars show activity in the presence of hormone. The activity of AR in the absence of a coactivator and in the presence of mibolerone was set at 100%, and other values are expressed relative to this. The values above each of the gray bars show the fold induction of hormone-dependent activity relative to hormone-independent activity for each condition. (B) AR AF2 activity is potentiated by full-length SRC1 in yeast. Yeast (W3031B) was cotransfected with the AR LBD fused to a heterologous DBD, a lacZ reporter, and full-length SRC1a, SRC1e, or RIP140 and incubated overnight in the presence and absence of 100 nM mibolerone (MB). β-Galactosidase activity was measured and normalized for protein content. The experiment was repeated on several individual transformants. Results of a representative example are shown. NH, no hormone.
FIG. 4
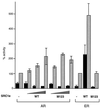
Leucine motifs are not required for potentiation of AR activity by SRC1e. COS-1 cells were transfected as described previously or with the ER expression vector pSG5-MOR and the estrogen-responsive reporter as previously described. Concentrations of coactivators used were 50, 100, and 200 ng for AR and 50 and 200 ng for ER. Activity was measured in the absence (black bars) or presence (gray bars) of 10 nM ligand. WT, wild type.
FIG. 5
Potentiation of the AR by SRC1 requires the glutamine-rich region, residues 1053 to 1123. (A) COS-1 cells were transfected with 50 or 200 ng of each deletion mutant of SRC1. CAT activity was measured in the absence (black bars) or presence (gray bars) of 10 nM mibolerone. (B) Alignment of the p160 coactivator proteins in the glutamine-rich region. Residues conserved across all three proteins are boxed.
FIG. 6
AR AF1 interacts with the glutamine-rich region of SRC1. (A) Interaction of full-length AR with fragments of SRC1 in the absence of ligand in yeast. Full-length AR fused to the lexA DBD was coexpressed in yeast (strain L40) with the VP16 activation domain alone (lane 1) or fused to fragments of SRC1 (lanes 2 to 5) in the presence of a _lexA_-responsive lacZ reporter. Yeast were incubated in liquid culture overnight, and β-galactosidase activity was assayed and corrected for protein content. (B) Interaction of AF1 with fragments of SRC1 in COS cells. SRC1 fragments fused to the Gal4 DBD were coexpressed in COS cells with a Gal4-responsive reporter and VP16 activation domain either alone (black bars) or fused to AR AF1 (hatched bars). The experiment was repeated several times, and results of a representative example are shown.
FIG. 7
Interaction of AF1 with SRC1 requires residues 1053 to 1123 of SRC1 and involves residues 360 to 494 of AF1 (TAU5). (A) Interaction of SRC1 residues 989 to 1240 with the AF1 region of AR requires the glutamine-rich region of SRC1 but not the N-terminal 35 amino acids of AR. The SRC1 fragment, with or without residues 1053 to 1123, was fused to the lexA DBD and expressed in yeast (L40) with the Gal4 activation domain alone (lanes 1, 4, and 7) or fused to AF1 (lanes 2, 5, and 6) or an AF1 deletion mutant (Δ1–35) (lanes 3, 6, and 9) (with the vector pGAF424). Yeast cells were grown and assayed as described in the text. (B) AR interacts in vitro with the glutamine-rich region of SRC1. GST fusion proteins, coupled to Sepharose beads, were incubated with in vitro-translated [35S]methionine-labeled full-length AR. After being washed extensively, samples were boiled and run on sodium dodecyl sulfate–8% polyacrylamide gel, which was fixed and dried, and the bound labeled protein was visualized by autoradiography. (C) TAU5 is able to interact with SRC1, dependent on the region from residues 1053 to 1123, but is not sufficient for maximal interaction. SRC1 fragments were used as bait, and the interaction with AF1 or with a fragment of AF1 comprising the TAU5 region fused to the Gal4 activation domain (in the pACT2 vector) was measured. (D) The TAU5 region of AF1 is sufficient for SRC1 potentiation of the AR. The Δ1–360 mutant (represented at the top of the figure) was cotransfected into COS cells with SRC1 mutants as shown, and activities were measured in the absence (black bars) and presence (gray bars) of hormone as described in the text. WT, wild type.
FIG. 8
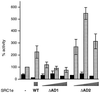
Potentiation of AR activity by SRC1 requires AD1 but not AD2. COS-1 cells were transfected as described for Fig. 1 with either 200 ng of wild-type SRC1e (WT) or 50, 100, or 200 ng of the deletion mutants, in the absence (black bars) or presence (gray bars) of 10 nM mibolerone.
Similar articles
- Mechanisms of androgen receptor signalling via steroid receptor coactivator-1 in prostate.
Powell SM, Christiaens V, Voulgaraki D, Waxman J, Claessens F, Bevan CL. Powell SM, et al. Endocr Relat Cancer. 2004 Mar;11(1):117-30. doi: 10.1677/erc.0.0110117. Endocr Relat Cancer. 2004. PMID: 15027889 - Analysis of the steroid receptor coactivator 1 (SRC1)-CREB binding protein interaction interface and its importance for the function of SRC1.
Sheppard HM, Harries JC, Hussain S, Bevan C, Heery DM. Sheppard HM, et al. Mol Cell Biol. 2001 Jan;21(1):39-50. doi: 10.1128/MCB.21.1.39-50.2001. Mol Cell Biol. 2001. PMID: 11113179 Free PMC article. - Electrostatic modulation in steroid receptor recruitment of LXXLL and FXXLF motifs.
He B, Wilson EM. He B, et al. Mol Cell Biol. 2003 Mar;23(6):2135-50. doi: 10.1128/MCB.23.6.2135-2150.2003. Mol Cell Biol. 2003. PMID: 12612084 Free PMC article. - Expression and function of androgen receptor coactivators in prostate cancer.
Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Culig Z, et al. J Steroid Biochem Mol Biol. 2004 Nov;92(4):265-71. doi: 10.1016/j.jsbmb.2004.10.003. Epub 2004 Dec 19. J Steroid Biochem Mol Biol. 2004. PMID: 15663989 Review. - Identification and characterization of androgen receptor associated coregulators in prostate cancer cells.
Sampson ER, Yeh SY, Chang HC, Tsai MY, Wang X, Ting HJ, Chang C. Sampson ER, et al. J Biol Regul Homeost Agents. 2001 Apr-Jun;15(2):123-9. J Biol Regul Homeost Agents. 2001. PMID: 11501969 Review.
Cited by
- Androgen receptor gene rearrangements: new perspectives on prostate cancer progression.
Brand LJ, Dehm SM. Brand LJ, et al. Curr Drug Targets. 2013 Apr;14(4):441-9. doi: 10.2174/1389450111314040005. Curr Drug Targets. 2013. PMID: 23410127 Free PMC article. Review. - AIB1 polymorphisms with breast cancer susceptibility: a pooled analysis of variation in BRCA1/2 mutation carriers and non-carriers.
Zhang Y, Huang M, Zhu Z. Zhang Y, et al. Mol Biol Rep. 2012 Jun;39(6):6881-6. doi: 10.1007/s11033-012-1514-2. Epub 2012 Feb 4. Mol Biol Rep. 2012. PMID: 22307791 - Modulation of androgen receptor transactivation by the SWI3-related gene product (SRG3) in multiple ways.
Hong CY, Suh JH, Kim K, Gong EY, Jeon SH, Ko M, Seong RH, Kwon HB, Lee K. Hong CY, et al. Mol Cell Biol. 2005 Jun;25(12):4841-52. doi: 10.1128/MCB.25.12.4841-4852.2005. Mol Cell Biol. 2005. PMID: 15923603 Free PMC article. - MOZ-TIF2 repression of nuclear receptor-mediated transcription requires multiple domains in MOZ and in the CID domain of TIF2.
Yin H, Glass J, Blanchard KL. Yin H, et al. Mol Cancer. 2007 Aug 13;6:51. doi: 10.1186/1476-4598-6-51. Mol Cancer. 2007. PMID: 17697320 Free PMC article. - AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR.
Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. Li P, et al. Mol Cell Biol. 2003 Jan;23(1):104-18. doi: 10.1128/MCB.23.1.104-118.2003. Mol Cell Biol. 2003. PMID: 12482965 Free PMC article.
References
- Alen P, Claessens F, Schoenmakers E, Swinnen J V, Verhoeven G, Rombauts W, Peeters B. Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1a with multiple steroid receptors and identification of an internally deleted ELE1β isoform. Mol Endocrinol. 1999;13:117–128. - PubMed
- Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X-Y, Sauter G, Kallioniemi O-P, Trent J M, Meltzer P S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. - PubMed
- Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;1996:641–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous