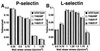Tyrosine replacement in P-selectin glycoprotein ligand-1 affects distinct kinetic and mechanical properties of bonds with P- and L-selectin - PubMed (original) (raw)
Tyrosine replacement in P-selectin glycoprotein ligand-1 affects distinct kinetic and mechanical properties of bonds with P- and L-selectin
V Ramachandran et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Selectins are adhesion molecules that initiate tethering and rolling of leukocytes on the vessel wall. Rolling requires rapid formation and breakage of selectin-ligand bonds that must have mechanical strength to resist premature dissociation by the forces applied in shear flow. P- and L-selectin bind to the N-terminal region of P-selectin glycoprotein ligand-1 (PSGL-1), a mucin on leukocytes. To define determinants on PSGL-1 that contribute to the kinetic and mechanical properties of bonds with selectins, we compared rolling of transfected preB cells expressing P- or L-selectin on transfected cell monolayers expressing wild-type PSGL-1 or PSGL-1 constructs with substitutions in targeted N-terminal residues. Rolling through P- or L-selectin required a Thr or Ser at a specific position on PSGL-1, the attachment site for an essential O-glycan, but required only one of three nearby Tyr residues, which are sites for Tyr-SO(3) formation. The adhesive strengths and numbers of cells rolling through P- or L-selectin were similar on wild-type PSGL-1 and on each of the three PSGL-1 constructs containing only a single Tyr. However, the cells rolled more irregularly on the single-Tyr forms of PSGL-1. Analysis of the lifetimes of transient tethers on limiting densities of PSGL-1 revealed that L-selectin dissociated faster from single-Tyr than wild-type PSGL-1 at all shears examined. In sharp contrast, P-selectin dissociated faster from single-Tyr than wild-type PSGL-1 at higher shear but not at lower shear. Thus, tyrosine replacements in PSGL-1 affect distinct kinetic and mechanical properties of bonds with P- and L-selectin.
Figures
Figure 1
Schematic diagram of human PSGL-1 constructs. (A) Diagram of the domains in wild-type PSGL-1, which self-associates to form homodimers in the membrane. The residue number that begins each region of the extracellular and transmembrane domains is indicated. The N terminus of mature PSGL-1 begins at residue 42, immediately after the propeptide. The sequence of the first 21 amino acids of mature PSGL-1 is listed. The epitope for the blocking anti-PSGL-1 mAb PL1 (30) is bracketed. Shown are the three consensus tyrosine sulfation sites at residues 46, 48, and 51, as well as Thr-57, a site for attachment for an O-glycan. (B) Sequences of the amino acid substitutions in the full-length PSGL-1 constructs.
Figure 2
Accumulation of rolling cells expressing P- or L-selectin on PSGL-1 constructs. L1–2 cells expressing human P-selectin (A_and C) or L-selectin (B and_D) were perfused at the indicated wall shear stress over CHO cell monolayers expressing Fuc-TVII, C2GnT, and the indicated PSGL-1 construct. After 4 min of perfusion, the number of L1–2 cells rolling on the monolayer was quantified. The data represent the mean ± SD of five experiments.
Figure 3
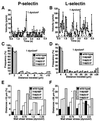
Rate of tethering of cells expressing P- or L-selectin to wild-type or single-Tyr PSGL-1. Cells expressing P-selectin (A) or L-selectin (B) were perfused over each PSGL-1 construct at the indicated wall shear stress. The number of cells that tethered to the monolayer over a 30-s interval was quantified and normalized by dividing by the number of cells delivered across the field of view in the focal plane of the monolayer. The data represent the mean ± SD of four experiments.
Figure 4
Kinetics of rolling of cells expressing P- or L-selectin on wild-type or single-Tyr PSGL-1. (A and B) Frame-by-frame velocities of representative L1–2 cells expressing P- or L-selectin rolling on wild-type PSGL-1 at 1 dyn/cm2. The dashed line represents the velocity threshold used to separate paused from rolling cells. (C and D) Distribution of travel distances for cells rolling on wild-type or single-Tyr PSGL-1 at 1 dyn/cm2. At least 20 L1–2 cells were tracked for up to 5 s for a total observation time of ≥100 s. The travel distance of a rolling cell was defined as the distance spanned during one continuous sequence of velocities >6 μm/s. The bars represent an estimate of the cumulative error for the measurements. (E and F) Frequency of large distances traveled (>10 μm for P-selectin or >28 μm for L-selectin) for L1–2 cells rolling on wild-type or single-Tyr PSGL-1 as a function of wall shear stress.
Figure 5
Kinetics of dissociation and mechanical strength of transient tethers of P- or L-selectin-expressing cells to wild-type or single-Tyr PSGL-1. (A and B) Representative first-order dissociation kinetics for transient tethers of L1–2 cells expressing P- or L-selectin to each PSGL-1 construct at the indicated wall shear stress. For each set of tether lifetimes, the line is the least squares fit, and the slope is −_k_off for the selectin–PSGL-1 bond. (C and D) Effect of increased wall shear stress and force on the tether bond on dissociation kinetics of P- or L-selectin with wild-type or single-Tyr PSGL-1. Each group of points represents a single wall shear stress from each of five independent experiments. The points were displaced horizontally so that all points could be seen. The data were fit to the Bell equation (24).
Similar articles
- Molecular basis of leukocyte rolling on PSGL-1. Predominant role of core-2 O-glycans and of tyrosine sulfate residue 51.
Bernimoulin MP, Zeng XL, Abbal C, Giraud S, Martinez M, Michielin O, Schapira M, Spertini O. Bernimoulin MP, et al. J Biol Chem. 2003 Jan 3;278(1):37-47. doi: 10.1074/jbc.M204360200. Epub 2002 Oct 25. J Biol Chem. 2003. PMID: 12403782 - Dimerization of a selectin and its ligand stabilizes cell rolling and enhances tether strength in shear flow.
Ramachandran V, Yago T, Epperson TK, Kobzdej MM, Nollert MU, Cummings RD, Zhu C, McEver RP. Ramachandran V, et al. Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10166-71. doi: 10.1073/pnas.171248098. Epub 2001 Jul 31. Proc Natl Acad Sci U S A. 2001. PMID: 11481445 Free PMC article. - Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin.
Leppänen A, Yago T, Otto VI, McEver RP, Cummings RD. Leppänen A, et al. J Biol Chem. 2003 Jul 18;278(29):26391-400. doi: 10.1074/jbc.M303551200. Epub 2003 May 7. J Biol Chem. 2003. PMID: 12736247 - PSGL-1 function in immunity and steady state homeostasis.
Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. Carlow DA, et al. Immunol Rev. 2009 Jul;230(1):75-96. doi: 10.1111/j.1600-065X.2009.00797.x. Immunol Rev. 2009. PMID: 19594630 Review. - Structure and function of P-selectin glycoprotein ligand-1.
Moore KL. Moore KL. Leuk Lymphoma. 1998 Mar;29(1-2):1-15. doi: 10.3109/10428199809058377. Leuk Lymphoma. 1998. PMID: 9638971 Review.
Cited by
- Highly Biocompatible Functionalized Layer-by-Layer Ginger Lipid Nano Vectors Targeting P-selectin for Delivery of Doxorubicin to Treat Colon Cancer.
Zhang M, Yang C, Yan X, Sung J, Garg P, Merlin D. Zhang M, et al. Adv Ther (Weinh). 2019 Dec;2(12):1900129. doi: 10.1002/adtp.201900129. Epub 2019 Sep 18. Adv Ther (Weinh). 2019. PMID: 33043129 Free PMC article. - Bistability of cell adhesion in shear flow.
Efremov A, Cao J. Efremov A, et al. Biophys J. 2011 Sep 7;101(5):1032-40. doi: 10.1016/j.bpj.2011.07.026. Biophys J. 2011. PMID: 21889439 Free PMC article. - Functional binding of E-selectin to its ligands is enhanced by structural features beyond its lectin domain.
Aleisa FA, Sakashita K, Lee JM, AbuSamra DB, Al Alwan B, Nozue S, Tehseen M, Hamdan SM, Habuchi S, Kusakabe T, Merzaban JS. Aleisa FA, et al. J Biol Chem. 2020 Mar 13;295(11):3719-3733. doi: 10.1074/jbc.RA119.010910. Epub 2020 Jan 16. J Biol Chem. 2020. PMID: 31949047 Free PMC article. - P-selectin glycoprotein ligand-1 forms dimeric interactions with E-selectin but monomeric interactions with L-selectin on cell surfaces.
Zhang Y, Jiang N, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C. Zhang Y, et al. PLoS One. 2013;8(2):e57202. doi: 10.1371/journal.pone.0057202. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451187 Free PMC article. - Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow.
Yago T, Leppänen A, Qiu H, Marcus WD, Nollert MU, Zhu C, Cummings RD, McEver RP. Yago T, et al. J Cell Biol. 2002 Aug 19;158(4):787-99. doi: 10.1083/jcb.200204041. Epub 2002 Aug 12. J Cell Biol. 2002. PMID: 12177042 Free PMC article.
References
- McEver R P, Moore K L, Cummings R D. J Biol Chem. 1995;270:11025–11028. - PubMed
- Kansas G S. Blood. 1996;88:3259–3287. - PubMed
- Lawrence M B, Springer T A. Cell. 1991;65:859–873. - PubMed
- Alon R, Hammer D A, Springer T A. Nature (London) 1995;374:539–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources