Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming - PubMed (original) (raw)
Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming
L S Jouaville et al. Proc Natl Acad Sci U S A. 1999.
Abstract
In recent years, mitochondria have emerged as important targets of agonist-dependent increases in cytosolic Ca(2+) concentration. Here, we analyzed the significance of Ca(2+) signals for the modulation of organelle function by directly measuring mitochondrial and cytosolic ATP levels ([ATP](m) and [ATP](c), respectively) with specifically targeted chimeras of the ATP-dependent photoprotein luciferase. In both HeLa cells and primary cultures of skeletal myotubes, stimulation with agonists evoking cytosolic and mitochondrial Ca(2+) signals caused increases in [ATP](m) and [ATP](c) that depended on two parameters: (i) the amplitude of the Ca(2+) rise in the mitochondrial matrix, and (ii) the availability of mitochondrial substrates. Moreover, the Ca(2+) elevation induced a long-lasting priming that persisted long after agonist washout and caused a major increase in [ATP](m) upon addition of oxidative substrates. These results demonstrate a direct role of mitochondrial Ca(2+) in driving ATP production and unravel a form of cellular memory that allows a prolonged metabolic activation in stimulated cells.
Figures
Figure 1
(A) Schematic map of the chimeric mitochondrial luciferase. Lines and bars indicate the noncoding and coding regions [gray, cytochrome c oxidase subunit VIII (COX8); black, HA1; white, luciferase], respectively. Details of the construction strategy are given in Material and Methods. (B) Immunofluorescence image of HeLa cells transiently transfected with mtLuc and stained with the anti-HA1 mAb.
Figure 2
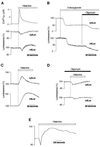
[ATP]m and [ATP]c changes elicited by Ca2+ signals in HeLa cells perfused with either glycolytic (A and B) or oxidative (C_and D) substrates. Measurements were carried out on HeLa cells transiently transfected with cytLuc, mtLuc, or mtAEQ. (A) Effects of histamine (100 μM, white bar) on [Ca2+]m, [ATP]c (cytLuc), and [ATP]m (mtLuc) in HeLa cells perfused with glucose as metabolic substrate. (B) Inhibition of mitochondrial ATP synthesis with oligomycin (5 μM) decreases [ATP]c and [ATP]m only after inhibition of glycolysis with 2-deoxyglucose (5 mM). (C) When HeLa cells are perfused with pyruvate and lactate instead of glucose, histamine provokes major increases in [ATP]c and [ATP]m. (D) Perfusion with oligomycin (5 μM) suppresses the histamine-dependent [ATP]c and [ATP]m rises observed when HeLa cells are perfused with pyruvate and lactate (see_C). (E) Agonist stimulation causes an increase in membrane potential ΔΨm as measured in pyruvate and lactate-perfused HeLa cells loaded with the mitochondrial potential-sensitive dye tetramethylrhodamine ethyl ester (10 nM) through changes in fluorescence (arbitrary units) (26). mtLuc and cytLuc luminescence data are expressed as a percentage of the initial value. Measurements of [Ca2+]m, [ATP]c, and [ATP]m were performed in parallel batches of cells transiently transfected with mtAEQ, cytLuc, and mtLuc.
Figure 3
[ATP]m increase triggered by KCl challenge of primary cultures of myotubes. (A) Myotubes were perfused with glucose as metabolic substrate. Simultaneous [Ca2+]m changes triggered by KCl are also shown (gray trace). (B) Perfusion of myotubes with pyruvate and lactate instead of glucose increases both kinetics and peak value of the [ATP]m rise. (C) Pretreatment of myotubes with atractyloside (40 μM) reduces the KCl-induced [ATP]m rise. Throughout the experiments shown in this figure, cells were perfused in Krebs–Ringer buffer with pyruvate and lactate as metabolic substrate.
Figure 4
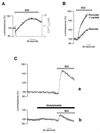
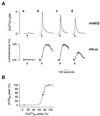
[Ca2+]m dependency of the [ATP]m rise induced in HeLa cells by histamine. (A) Histamine-dependent [Ca2+]m and [ATP]m changes at different states of filling of the endoplasmic reticulum store. Throughout the experiments shown, the cells were perfused in Krebs–Ringer buffer with pyruvate and lactate as metabolic substrate, and, where indicated (arrow), challenged with histamine (100 μM). (a) Cells were loaded, before the experiment, with BAPTA-AM, as specified in the text. (b–d) The effects of increased time of incubation with Krebs–Ringer buffer supplemented with EGTA (1 mM) (b, control; c, 90 s; d, 300 s before histamine stimulation) on [Ca2+]m rise (mtAEQ) and [ATP]mrise (mtLuc). (B) Percentage of the [ATP]mpeak maximum as a function of the percentage of the [Ca2+]m peak. Values are derived from experiments shown in A. Extension to the origin of the_y_ axis is calculated from the experiments with the Ca2+ buffer BAPTA-AM (○).
Figure 5
Effects of prestimulation of HeLa cells with histamine on the subsequent response of [ATP]m and [ATP]c to a change in metabolic substrate (A and_B_). Shift from glucose to pyruvate and lactate as metabolic substrate in the perfusion medium produces a transient rise in [ATP]m (mtLuc) and [ATP]c (cytLuc) (A) that is increased when cells are stimulated previously with histamine (100 μM) (B), while no further changes in [Ca2+]m are observed (mtAEQ). (C) Substrate-induced [ATP]m peak maximum as a function of time after histamine removal.
Similar articles
- Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic β cells.
Tarasov AI, Semplici F, Li D, Rizzuto R, Ravier MA, Gilon P, Rutter GA. Tarasov AI, et al. Pflugers Arch. 2013 Apr;465(4):543-54. doi: 10.1007/s00424-012-1177-9. Epub 2012 Nov 14. Pflugers Arch. 2013. PMID: 23149488 Free PMC article. - Mitochondrial priming modifies Ca2+ oscillations and insulin secretion in pancreatic islets.
Ainscow EK, Rutter GA. Ainscow EK, et al. Biochem J. 2001 Jan 15;353(Pt 2):175-80. doi: 10.1042/0264-6021:3530175. Biochem J. 2001. PMID: 11139378 Free PMC article. - Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations.
Poburko D, Santo-Domingo J, Demaurex N. Poburko D, et al. J Biol Chem. 2011 Apr 1;286(13):11672-84. doi: 10.1074/jbc.M110.159962. Epub 2011 Jan 11. J Biol Chem. 2011. PMID: 21224385 Free PMC article. - Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells.
Griffiths EJ, Rutter GA. Griffiths EJ, et al. Biochim Biophys Acta. 2009 Nov;1787(11):1324-33. doi: 10.1016/j.bbabio.2009.01.019. Epub 2009 Feb 3. Biochim Biophys Acta. 2009. PMID: 19366607 Review. - Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs).
Rueda CB, Llorente-Folch I, Traba J, Amigo I, Gonzalez-Sanchez P, Contreras L, Juaristi I, Martinez-Valero P, Pardo B, Del Arco A, Satrustegui J. Rueda CB, et al. Biochim Biophys Acta. 2016 Aug;1857(8):1158-1166. doi: 10.1016/j.bbabio.2016.04.003. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060251 Review.
Cited by
- The Potential of Small Molecules to Modulate the Mitochondria-Endoplasmic Reticulum Interplay in Alzheimer's Disease.
Dentoni G, Castro-Aldrete L, Naia L, Ankarcrona M. Dentoni G, et al. Front Cell Dev Biol. 2022 Aug 26;10:920228. doi: 10.3389/fcell.2022.920228. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092728 Free PMC article. Review. - Age-Related Changes in Axonal and Mitochondrial Ultrastructure and Function in White Matter.
Stahon KE, Bastian C, Griffith S, Kidd GJ, Brunet S, Baltan S. Stahon KE, et al. J Neurosci. 2016 Sep 28;36(39):9990-10001. doi: 10.1523/JNEUROSCI.1316-16.2016. Epub 2016 Sep 28. J Neurosci. 2016. PMID: 27683897 Free PMC article. - Spatiotemporal correlations between cytosolic and mitochondrial Ca(2+) signals using a novel red-shifted mitochondrial targeted cameleon.
Waldeck-Weiermair M, Alam MR, Khan MJ, Deak AT, Vishnu N, Karsten F, Imamura H, Graier WF, Malli R. Waldeck-Weiermair M, et al. PLoS One. 2012;7(9):e45917. doi: 10.1371/journal.pone.0045917. Epub 2012 Sep 21. PLoS One. 2012. PMID: 23029314 Free PMC article. - Inositol 1,4,5-trisphosphate receptor type 3 plays a protective role in hepatocytes during hepatic ischemia-reperfusion injury.
Lima Filho ACM, França A, Florentino RM, Dos Santos ML, de Oliveira Lemos F, Missiaggia DG, Fonseca RC, Gustavo Oliveira A, Ananthanarayanan M, Guerra MT, de Castro Fonseca M, Vidigal PVT, Lima CX, Nathanson MH, Fatima Leite M. Lima Filho ACM, et al. Cell Calcium. 2020 Nov;91:102264. doi: 10.1016/j.ceca.2020.102264. Epub 2020 Aug 11. Cell Calcium. 2020. PMID: 32957029 Free PMC article. - Cell via Cell Viability Assay Changes Cellular Metabolic Characteristics by Intervening with Glycolysis and Pentose Phosphate Pathway.
Fan J, Schiemer T, Vaska A, Jahed V, Klavins K. Fan J, et al. Chem Res Toxicol. 2024 Feb 19;37(2):208-211. doi: 10.1021/acs.chemrestox.3c00339. Epub 2024 Jan 8. Chem Res Toxicol. 2024. PMID: 38191130 Free PMC article.
References
- Rizzuto R, Simpson A W, Brini M, Pozzan T. Nature (London) 1992;358:325–327. - PubMed
- Jouaville L S, Ichas F, Holmuhamedov E L, Camacho P, Lechleiter J D. Nature (London) 1995;377:348–441. - PubMed
- Budd S L, Nicholls D G. J Neurochem. 1996;66:403–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous