Genes of the sbo-alb locus of Bacillus subtilis are required for production of the antilisterial bacteriocin subtilosin - PubMed (original) (raw)
Genes of the sbo-alb locus of Bacillus subtilis are required for production of the antilisterial bacteriocin subtilosin
G Zheng et al. J Bacteriol. 1999 Dec.
Abstract
Bacillus subtilis JH642 and a wild strain of B. subtilis called 22a both produce an antilisterial peptide that can be purified by anion-exchange and gel filtration chromatography. Amino acid analysis confirmed that the substance was the cyclic bacteriocin subtilosin. A mutant defective in production of the substance was isolated from a plasmid gene disruption library. The plasmid insertion conferring the antilisterial-peptide-negative phenotype was located in a seven-gene operon (alb, for antilisterial bacteriocin) residing immediately downstream from the sbo gene, which encodes the precursor of subtilosin. An insertion mutation in the sbo gene also conferred loss of antilisterial activity. Comparison of the presubtilosin and mature subtilosin sequences suggested that certain residues undergo unusual posttranslational modifications unlike those occurring during the synthesis of class I (lantibiotic) or some class II bacteriocins. The putative products of the genes of the operon identified show similarities to peptidases and transport proteins that may function in processing and export. Two alb gene products resemble proteins that function in pyrroloquinoline quinone biosynthesis. The use of lacZ-alb and lacZ-sbo gene fusions, along with primer extension analysis, revealed that the sbo-alb genes are transcribed from a major promoter, residing upstream of sbo, that is very likely utilized by the sigma(A) form of RNA polymerase. The sbo and alb genes are negatively regulated by the global transition state regulator AbrB and are also under positive autoregulation that is not mediated by the subtilosin peptide but instead requires one or more of the alb gene products.
Figures
FIG. 1
(A) Proposed structure of subtilosin (1). The boldfaced N and C mark the N and C termini of the prosubtilosin peptide (presubtilosin leader peptide removed), respectively. The proposed link between Glu31 and Cys21 (1) is shown. The asterisks on either side of an amino acid indicate residues that have likely undergone chemical modification. SS, disulfide link between Cys12 and Cys15. (B) Amino acid sequence of the presubtilosin peptide, deduced from the nucleotide sequence of the sbo coding region.
FIG. 2
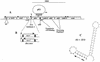
(A) Organization of the alb operon and flanking chromosomal regions of the B. subtilis chromosome. Rectangular boxes indicate the positions of genes in the alb region. The arrows above the boxes indicate the transcription units deduced by the Bacillus genome sequencing project (30). Also shown are the locations of the alb operon and the sbo gene, as well as the genetically linked fnr gene and the fnr::spc insertion mutation (used to demonstrate linkage) (31). The integrative plasmid pE1 and the site of its recombination with the chromosome, which causes disruption of the alb operon, are shown. T, putative transcription termination site. (B) Location of the sbo::neo-1 and sbo::neo-2 insertions. (C) The stem-loop structure predicted from inspection of the sbo-albA intergenic-region sequence that overlaps with the 3′ end of the sbo gene (indicated in boldface). Also shown is the free energy of secondary-structure formation, ΔG.
FIG. 3
Antilisterial substance produced by B. subtilis JH642 and 22a. (A) The strains are shown as colonies that were overlayed with soft agar containing a suspension of L. monocytogenes cells. Growth of Listeria is inhibited by wild-type (WT) JH642 and by 22a but not by the sbo and alb mutants of these organisms. Some slight inhibition is observed around the colonies of the 22a sbo and alb mutant cells. (B) A Tricine-SDS-PAGE gel that is stained with Coomassie blue shows the antilisterial peptide produced in WT JH642 but not present in cultures of strains ORB3148 (sbo) and ORB3146 (alb). (C) A bioautograph (see Materials and Methods) of the gel in panel B, showing the antilisterial activity of the peptide and the absence of activity in the lanes containing the sbo and alb mutant culture extracts.
FIG. 4
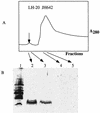
(A) Profile of fractions collected from an LH-20 size exclusion column onto which a methanol extract of concentrated JH642 culture supernatant was applied. A small absorbance peak (λ = 280) (arrow) contains the putative subtilosin peptide, as shown by Tricine-SDS-PAGE (B).
FIG. 5
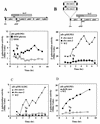
Expression of an alb-lacZ fusion constructed by creation of the alb::pMUPE1 insertion. Cultures were grown in DSM or DSM-G, and 1-ml samples were collected at either 30-min or 1-h intervals. β-Galactosidase activity was determined and plotted versus time. _T_0 indicates the end of the exponential growth phase. (A) Map of the alb operon and location of the lacZ fusion generated by recombination between the DNA of the alb locus and pMUPE1. Below the map is the expression profile of alb-lacZ in cells of strain ORB3147 grown in DSM and DSM-G. (B) Location of the sbo::neo-1 and sbo::neo-2 insertions with respect to the alb::pMUPE1 lacZ fusion. Also shown below the map is the expression profile of alb-lacZ in strains ORB3152 (sbo::neo-2) and ORB3153 (sbo::neo-1). (C) Expression profile of the albG-lacZ of strain ORB3231 (alb::pMUALBG) and its sbo::neo-1 (ORB3284) and sbo::neo-2 (ORB3230) mutant derivatives. (D) Effect of an abrB::neo insertion on the expression profile of alb-lacZ (alb::pMUPE1).
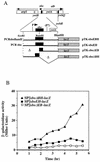
FIG. 6
(A) A fragment containing the sbo gene and the 5′ end of the alb operon was obtained by PCR and inserted into the promoter probe plasmid pTKlac (24). The _Eco_RI-_Bam_HI fragment bearing the putative sbo promoter region was deleted to create an alb-lacZ (sboΔEB-lacZ) fusion, and the _Bam_HI-Hin_dIII fragment was deleted to create an sbo-lacZ (sboΔBH-lacZ) fusion. Additionally, the Eco_RI-Hin_dIII fragment containing the entire sbo gene and flanking DNA was inserted into pTKlac to create sboEH-lacZ, while the fragment containing the PCR-generated sboEBH allele was inserted into pTKlac to create the sboEBH-lacZ construct. The fusions thus constructed were inserted into the SPβ prophage of B. subtilis, using a published protocol. (B) Expression of the phage-borne fusions in cells of strains ORB3158 (SPβ_sboΔEB-lacZ), ORB3162 (SPβ–_sboΔBH-lacZ), and ORB3166 (SPβ_sboEH-lacZ) grown in DSM-G. _T_0, the time at which exponential growth ceases, is indicated by an arrow. (C) Primer extension analysis of RNA from JH642 and ZB449 (abrB703) cells. RNA was purified from cells of cultures grown to _T_2 in DSM-G. On the left is the autoradiograph showing the sequence pattern of a dideoxynucleotide sequencing reaction containing primer osboP4 and pTK-sboEH DNA. Lane 1, primer extension product from a reaction containing JH642 RNA and the osboP4 oligonucleotide; lane 2, primer extension product of the reaction of ZB449 RNA and osboP4 primer. The arrow indicates the primer extension products and the proposed start site of transcription in the sequence at the left. On the right is the nucleotide sequence of the sbo promoter region, with the ATG start codon and Shine-Delgarno (SD) sequence shown. The putative −10 and −35 regions of the sbo promoter are underlined. An asterisk marks the transcriptional start site.
FIG. 6
(A) A fragment containing the sbo gene and the 5′ end of the alb operon was obtained by PCR and inserted into the promoter probe plasmid pTKlac (24). The _Eco_RI-_Bam_HI fragment bearing the putative sbo promoter region was deleted to create an alb-lacZ (sboΔEB-lacZ) fusion, and the _Bam_HI-Hin_dIII fragment was deleted to create an sbo-lacZ (sboΔBH-lacZ) fusion. Additionally, the Eco_RI-Hin_dIII fragment containing the entire sbo gene and flanking DNA was inserted into pTKlac to create sboEH-lacZ, while the fragment containing the PCR-generated sboEBH allele was inserted into pTKlac to create the sboEBH-lacZ construct. The fusions thus constructed were inserted into the SPβ prophage of B. subtilis, using a published protocol. (B) Expression of the phage-borne fusions in cells of strains ORB3158 (SPβ_sboΔEB-lacZ), ORB3162 (SPβ–_sboΔBH-lacZ), and ORB3166 (SPβ_sboEH-lacZ) grown in DSM-G. _T_0, the time at which exponential growth ceases, is indicated by an arrow. (C) Primer extension analysis of RNA from JH642 and ZB449 (abrB703) cells. RNA was purified from cells of cultures grown to _T_2 in DSM-G. On the left is the autoradiograph showing the sequence pattern of a dideoxynucleotide sequencing reaction containing primer osboP4 and pTK-sboEH DNA. Lane 1, primer extension product from a reaction containing JH642 RNA and the osboP4 oligonucleotide; lane 2, primer extension product of the reaction of ZB449 RNA and osboP4 primer. The arrow indicates the primer extension products and the proposed start site of transcription in the sequence at the left. On the right is the nucleotide sequence of the sbo promoter region, with the ATG start codon and Shine-Delgarno (SD) sequence shown. The putative −10 and −35 regions of the sbo promoter are underlined. An asterisk marks the transcriptional start site.
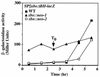
FIG. 7
alb_-dependent stimulation of sbo transcription. Strains ORB3162 (SPβ–_sboΔBH-lacZ), ORB3163 (sbo::neo-1 SPβ–sboΔBH-lacZ), and ORB3164 (sbo::neo-2 SPβ–sboΔBH-lacZ) were grown in DSM-G. The profiles of sboΔBH-lacZ expression in the three strains over the exponential and stationary phases of growth are shown. WT, wild type.
Similar articles
- Mutational analysis of the sbo-alb locus of Bacillus subtilis: identification of genes required for subtilosin production and immunity.
Zheng G, Hehn R, Zuber P. Zheng G, et al. J Bacteriol. 2000 Jun;182(11):3266-73. doi: 10.1128/JB.182.11.3266-3273.2000. J Bacteriol. 2000. PMID: 10809709 Free PMC article. - Dual control of sbo-alb operon expression by the Spo0 and ResDE systems of signal transduction under anaerobic conditions in Bacillus subtilis.
Nakano MM, Zheng G, Zuber P. Nakano MM, et al. J Bacteriol. 2000 Jun;182(11):3274-7. doi: 10.1128/JB.182.11.3274-3277.2000. J Bacteriol. 2000. PMID: 10809710 Free PMC article. - Subtilosin production by two Bacillus subtilis subspecies and variance of the sbo-alb cluster.
Stein T, Düsterhus S, Stroh A, Entian KD. Stein T, et al. Appl Environ Microbiol. 2004 Apr;70(4):2349-53. doi: 10.1128/AEM.70.4.2349-2353.2004. Appl Environ Microbiol. 2004. PMID: 15066831 Free PMC article. - Antibacterial activity and genotypic-phenotypic characteristics of bacteriocin-producing Bacillus subtilis KKU213: potential as a probiotic strain.
Khochamit N, Siripornadulsil S, Sukon P, Siripornadulsil W. Khochamit N, et al. Microbiol Res. 2015 Jan;170:36-50. doi: 10.1016/j.micres.2014.09.004. Epub 2014 Sep 22. Microbiol Res. 2015. PMID: 25440998 - Genetics of subtilin and nisin biosyntheses: biosynthesis of lantibiotics.
Entian KD, de Vos WM. Entian KD, et al. Antonie Van Leeuwenhoek. 1996 Feb;69(2):109-17. doi: 10.1007/BF00399416. Antonie Van Leeuwenhoek. 1996. PMID: 8775971 Review.
Cited by
- The radical SAM enzyme AlbA catalyzes thioether bond formation in subtilosin A.
Flühe L, Knappe TA, Gattner MJ, Schäfer A, Burghaus O, Linne U, Marahiel MA. Flühe L, et al. Nat Chem Biol. 2012 Feb 26;8(4):350-7. doi: 10.1038/nchembio.798. Nat Chem Biol. 2012. PMID: 22366720 - Mechanisms of action of ribosomally synthesized and posttranslationally modified peptides (RiPPs).
Cao L, Do T, Link AJ. Cao L, et al. J Ind Microbiol Biotechnol. 2021 Jun 4;48(3-4):kuab005. doi: 10.1093/jimb/kuab005. J Ind Microbiol Biotechnol. 2021. PMID: 33928382 Free PMC article. Review. - Involvement of ResE phosphatase activity in down-regulation of ResD-controlled genes in Bacillus subtilis during aerobic growth.
Nakano MM, Zhu Y. Nakano MM, et al. J Bacteriol. 2001 Mar;183(6):1938-44. doi: 10.1128/JB.183.6.1938-1944.2001. J Bacteriol. 2001. PMID: 11222591 Free PMC article. - Radical S-adenosylmethionine enzymes.
Broderick JB, Duffus BR, Duschene KS, Shepard EM. Broderick JB, et al. Chem Rev. 2014 Apr 23;114(8):4229-317. doi: 10.1021/cr4004709. Epub 2014 Jan 29. Chem Rev. 2014. PMID: 24476342 Free PMC article. Review. No abstract available. - Identification of a new Bacillus licheniformis strain producing a bacteriocin-like substance.
Guo Y, Yu Z, Xie J, Zhang R. Guo Y, et al. J Microbiol. 2012 Jun;50(3):452-8. doi: 10.1007/s12275-012-2051-3. Epub 2012 Jun 30. J Microbiol. 2012. PMID: 22752909
References
- Babasaki K, Takao T, Shimonishi Y, Kurahashi K. Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: isolation, structural analysis, and biogenesis. J Biochem. 1985;98:583–603. - PubMed
- Burbulys D, Trach K A, Hoch J A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. - PubMed
- de Vos W M, Kuipers O P, Roelof van der Meer J, Siezen R J. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by Gram-positive bacteria. Mol Microbiol. 1995;17:427–437. - PubMed
- Dubnau D, Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971;56:209–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous