A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis - PubMed (original) (raw)
A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis
J R Morgan et al. J Neurosci. 1999.
Abstract
We have used the squid giant synapse to determine whether clathrin assembly by AP180 is important for synaptic vesicle endocytosis. The squid homolog of AP180 encodes a 751 amino acid protein with 40% sequence identity to mouse AP180. Alignment of squid AP180 with other AP180 homologs shows that amino acid identity was highest in the N-terminal inositide-binding domain of the protein and weakest in the C-terminal clathrin assembly domain. Recombinant squid AP180 was able to assemble clathrin in vitro, suggesting a conserved three-dimensional structure that mediates clathrin assembly despite the divergent primary sequence of the C-terminal domain. Microinjection of the C-terminal domains of either mouse or squid AP180 into the giant presynaptic terminal of squid enhanced synaptic transmission. Conversely, a peptide from the C-terminal domain of squid AP180 that inhibited clathrin assembly in vitro completely blocked synaptic transmission when it was injected into the giant presynaptic terminal. This inhibitory effect occurred over a time scale of minutes when the synapse was stimulated at low (0.03 Hz), physiological rates. Electron microscopic analysis revealed several structural changes consistent with the inhibition of synaptic vesicle endocytosis; peptide-injected terminals had far fewer synaptic vesicles, were depleted of coated vesicles, and had a larger plasma membrane perimeter than terminals injected with control solutions. In addition, the remaining synaptic vesicles were significantly larger in diameter. We conclude that the clathrin assembly domain of AP180 is important for synaptic vesicle recycling at physiological rates of activity and that assembly of clathrin by AP180 is necessary for maintaining a pool of releasable synaptic vesicles.
Figures
Fig. 1.
Amino acid sequence alignment of the AP180 family members. Residues present in two or more family members are highlighted in black.
Fig. 1.
Amino acid sequence alignment of the AP180 family members. Residues present in two or more family members are highlighted in black.
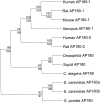
Fig. 2.
Phylogenetic relationships among the AP180 family members.
Fig. 3.
Squid GST-AP180 and squid GST-c45, but not GST, assemble clathrin as efficiently as bovine AP180. A–D, Clathrin assembly by squid GST-AP180 (A), squid GST-c45 (B), bovine AP180 (C), and GST (D).Points represent the mean of three to four independent experiments, and error bars indicate the SEM values. Half-maximal concentrations for clathrin assembly, determined by fits to a rectangular hyperbola function (solid lines), were 0.3 μ
m
for squid GST-AP180, 0.4 μ
m
for GST-c45, and 0.6 μ
m
for bovine AP180. The maximum amount of assembly ranged from 85 to 94% for these three proteins.E, Electron micrograph of clathrin coats assembled by squid GST-AP180.
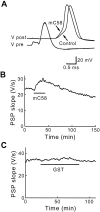
Fig. 4.
Presynaptic microinjection of the 58 kDa clathrin assembly domain of mouse AP180–1 (mC58) enhances transmission. A, Superimposed traces of presynaptic (_V_pre) and postsynaptic (_V_post) responses during microinjection of mC58. mC58 enhanced the postsynaptic responses, whereas the presynaptic response remained unchanged. _B,_Time course of response to the injection of mC58 (10 μ
m
; during bar). mC58 reversibly enhanced transmitter release when the synapse was stimulated at 0.03 Hz. C, GST alone had no effect on transmitter release.
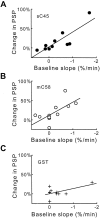
Fig. 5.
The effects of sC45 and mC58 depend on the physiological state of the synapse. A, B, When basal synaptic transmission (Baseline slope, measured as the rate of change of the PSP slope) was declining, the resulting enhancement in transmission (Change in PSP) seen with sC45 (A) and mC58 (B) is larger than when there is little or no decline in basal synaptic transmission. C, Injection of GST had little or no effect, even when basal synaptic transmission was declining rapidly. Data were fit by a linear function (solid lines).
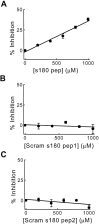
Fig. 6.
A peptide from the clathrin assembly domain of squid AP180 (s180 pep) inhibits clathrin assembly in vitro. A, The amount of inhibition of clathrin assembly depended on the concentration of s180 pep. Points represent the mean of three independent experiments, and error bars indicate SE.B, C, Two scrambled s180 peptides, Scram s180 pep1 and Scram s180 pep2, had no effect on clathrin assembly. Because none of the peptides inhibited by >50% over this concentration range, these data were fit by a linear function (solid lines).
Fig. 7.
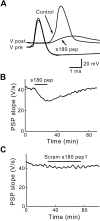
Sequence-specific inhibition of transmitter release by s180 pep. A, Superimposed traces of presynaptic and postsynaptic responses recorded before (Control) and during the injection of s180 pep. s180 pep reduced PSPs below the threshold for producing a postsynaptic action potential. B, The inhibitory effects of s180 pep were reversible after cessation of the peptide injection (during_bar_). C, Scram s180 pep1 had no effect on transmitter release.
Fig. 8.
Presynaptic terminals injected with s180 pep are depleted of synaptic vesicles. Compared with terminals injected with inert control solutions (A), s180 pep-injected terminals (B) had drastically fewer synaptic vesicles. Asterisks mark postsynaptic spines. Scale bar in A applies to both panels.
Fig. 9.
s180 pep inhibits endocytosis in the squid giant presynaptic terminal. A, Spatial distribution of synaptic vesicles in terminals injected with s180 pep or control solutions. Error bars indicate means and SE values for 376 active zones from two terminals injected with s180 pep and 209 active zones from three control terminals. B, Relative spatial distribution of synaptic vesicles, determined from the data in_A_ by dividing the means for s180 pep by those for control terminals. The dashed line indicates a ratio of 1, representing no effect of s180 pep. C, D, Mean number of synaptic vesicles (C) and coated vesicles (D) in terminals injected with s180 pep or control solution. E, Mean areas of membrane in synaptic vesicles (SV), coated vesicles (CV), and plasma membrane (PM) in sections taken from terminals injected with s180 pep or control solution. F–H, Distribution of synaptic vesicle (SV) diameters in terminals injected with control solution (F) or s180 pep (G). The curved lines indicate Gaussian functions fit to these distributions. _H,Superimposition of the two distributions, with s180 pep measurements in_black.
Similar articles
- A conserved clathrin assembly motif essential for synaptic vesicle endocytosis.
Morgan JR, Prasad K, Hao W, Augustine GJ, Lafer EM. Morgan JR, et al. J Neurosci. 2000 Dec 1;20(23):8667-76. doi: 10.1523/JNEUROSCI.20-23-08667.2000. J Neurosci. 2000. PMID: 11102472 Free PMC article. - Clathrin and synaptic vesicle endocytosis: studies at the squid giant synapse.
Augustine GJ, Morgan JR, Villalba-Galea CA, Jin S, Prasad K, Lafer EM. Augustine GJ, et al. Biochem Soc Trans. 2006 Feb;34(Pt 1):68-72. doi: 10.1042/BST0340068. Biochem Soc Trans. 2006. PMID: 16417485 Free PMC article. Review. - Endocytosis: an assembly protein for clathrin cages.
McMahon HT. McMahon HT. Curr Biol. 1999 May 6;9(9):R332-5. doi: 10.1016/s0960-9822(99)80206-1. Curr Biol. 1999. PMID: 10330371 Review. - Eps15 homology domain-NPF motif interactions regulate clathrin coat assembly during synaptic vesicle recycling.
Morgan JR, Prasad K, Jin S, Augustine GJ, Lafer EM. Morgan JR, et al. J Biol Chem. 2003 Aug 29;278(35):33583-92. doi: 10.1074/jbc.M304346200. Epub 2003 Jun 14. J Biol Chem. 2003. PMID: 12807910 - A novel all helix fold of the AP180 amino-terminal domain for phosphoinositide binding and clathrin assembly in synaptic vesicle endocytosis.
Mao Y, Chen J, Maynard JA, Zhang B, Quiocho FA. Mao Y, et al. Cell. 2001 Feb 9;104(3):433-40. doi: 10.1016/s0092-8674(01)00230-6. Cell. 2001. PMID: 11239400
Cited by
- Does clathrin pull the fission trigger?
Di Paolo G, De Camilli P. Di Paolo G, et al. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):4981-3. doi: 10.1073/pnas.0930650100. Epub 2003 Apr 18. Proc Natl Acad Sci U S A. 2003. PMID: 12704235 Free PMC article. No abstract available. - A conserved clathrin assembly motif essential for synaptic vesicle endocytosis.
Morgan JR, Prasad K, Hao W, Augustine GJ, Lafer EM. Morgan JR, et al. J Neurosci. 2000 Dec 1;20(23):8667-76. doi: 10.1523/JNEUROSCI.20-23-08667.2000. J Neurosci. 2000. PMID: 11102472 Free PMC article. - Neuronal activity and the expression of clathrin-assembly protein AP180.
Wu F, Mattson MP, Yao PJ. Wu F, et al. Biochem Biophys Res Commun. 2010 Nov 12;402(2):297-300. doi: 10.1016/j.bbrc.2010.10.018. Epub 2010 Oct 19. Biochem Biophys Res Commun. 2010. PMID: 20937255 Free PMC article. - A role for an Hsp70 nucleotide exchange factor in the regulation of synaptic vesicle endocytosis.
Morgan JR, Jiang J, Oliphint PA, Jin S, Gimenez LE, Busch DJ, Foldes AE, Zhuo Y, Sousa R, Lafer EM. Morgan JR, et al. J Neurosci. 2013 May 1;33(18):8009-21. doi: 10.1523/JNEUROSCI.4505-12.2013. J Neurosci. 2013. PMID: 23637191 Free PMC article. - Clathrin and synaptic vesicle endocytosis: studies at the squid giant synapse.
Augustine GJ, Morgan JR, Villalba-Galea CA, Jin S, Prasad K, Lafer EM. Augustine GJ, et al. Biochem Soc Trans. 2006 Feb;34(Pt 1):68-72. doi: 10.1042/BST0340068. Biochem Soc Trans. 2006. PMID: 16417485 Free PMC article. Review.
References
- Augustine GJ, Burns ME, DeBello WM, Pettit DL, Schweizer FE. Exocytosis: proteins and perturbations. Annu Rev Pharmacol Toxicol. 1996;36:659–701. - PubMed
- Bommert K, Charlton MP, DeBello WM, Chin GJ, Betz H, Augustine GJ. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993;363:163–165. - PubMed
- Burns ME, Augustine GJ. Functional studies of presynaptic proteins at the squid giant synapse. In: Bellen H, editor. Neurotransmitter release: frontiers in molecular biology. Oxford UP; New York: 1999. pp. 237–264.
Publication types
MeSH terms
Substances
Grants and funding
- NS21624/NS/NINDS NIH HHS/United States
- R56 NS029051/NS/NINDS NIH HHS/United States
- R01 NS029051/NS/NINDS NIH HHS/United States
- R01 NS021624/NS/NINDS NIH HHS/United States
- NS29051/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases