Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers - PubMed (original) (raw)
Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers
T L Horvath et al. J Neurosci. 1999.
Abstract
Distinct brain peptidergic circuits govern peripheral energy homeostasis and related behavior. Here we report that mitochondrial uncoupling protein 2 (UCP2) is expressed discretely in neurons involved in homeostatic regulation. UCP2 protein was associated with the mitochondria of neurons, predominantly in axons and axon terminals. UCP2-producing neurons were found to be the targets of peripheral hormones, including leptin and gonadal steroids, and the presence of UCP2 protein in axonal processes predicted increased local brain mitochondrial uncoupling activity and heat production. In the hypothalamus, perikarya producing corticotropin-releasing factor, vasopressin, oxytocin, and neuropeptide Y also expressed UCP2. Furthermore, axon terminals containing UCP2 innervated diverse hypothalamic neuronal populations. These cells included those producing orexin, melanin-concentrating hormone, and luteinizing hormone-releasing hormone. When c-fos-expressing cells were analyzed in the basal brain after either fasting or cold exposure, it was found that all activated neurons received a robust UCP2 input on their perikarya and proximal dendrites. Thus, our data suggest the novel concept that heat produced by axonal UCP2 modulates neurotransmission in homeostatic centers, thereby coordinating the activity of those brain circuits that regulate daily energy balance and related autonomic and endocrine processes.
Figures
Fig. 1.
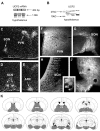
UCP2 mRNA and peptide expression in the brain.A, RT-PCR (top row) and Northern blot analyses of rat hypothalamic tissue demonstrates the expression of UCP2 mRNA. B, Western blot analysis of rat hypothalamic tissue revealed the expression of UCP2 peptide. Two major bands (arrows) were visible using the affinity-purified antisera: a band at 33 kDa that corresponds to the predicted molecular weight of UCP2 and another strong band at 23 kDa was also detected.C–E, In situ hybridization of UCP2 mRNA shows the concentration of labeled cells in four nuclei of the hypothalamus: the paraventricular (PVN), supraoptic (SON), suprachiasmatic (SCN), and arcuate nuclei (ARC).F–I, Although immunolabeling for UCP2 in the rat hypothalamus resulted in perikarya labeling of cells in the nuclei that contained UCP2 mRNA (SON, PVN, SCN, ARC), labeled cellular processes were abundant throughout the hypothalamus. For example, the median eminence (ME) that contains axonal fibers en route to the portal capillaries and the posterior lobe of the pituitary abundantly expressed UCP2. K, Schematic illustration (based on the rat brain atlas of Paxinos and Watson, 1997) of UCP2 (gray shaded areas) in the forebrain and diencephalon based on the _in situ_hybridization and immunocytochemistry studies.
Fig. 2.
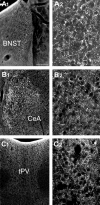
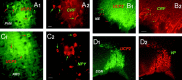
UCP2 in extrahypothalamic limbic sites.A1_–C_2, Light micrographs reveal the expression of UCP2 immunoreactivity in extrahypothalamic limbic sites. These areas included the bed nucleus of the stria terminalis (BNST; A1,A2), the central nucleus of the amygdala (CeA; B1,B2), and the thalamic paraventricular nucleus (tPV; C1,C2). The higher power magnifications (A2,B2, C2) demonstrate the robust expression of UCP2 in neuronal processes in each of these areas. Scale bars: A1, 100 μm; A2, 25 μm.
Fig. 3.
UCP2 in perikaryal mitochondria. Light (A) and electron (B) micrographs demonstrate UCP2 immunolabeling of perikaryal mitochondria (arrows) in the rat SCN. Immunoperoxidase is also associated with cytosol in close apposition to labeled mitochondria. Note the infolded nucleus of this SCN neuron. Scale bars:A, 10 μm; B, 1 μm.
Fig. 4.
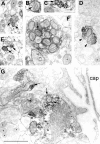
UCP2 in axonal mitochondria of the hypothalamus.A–G, Electron micrographs reveal the association of the UCP2 immunolabeling with mitochondria of different hypothalamic axons (arrows). Arrowheads in _D_point to a symmetrical synapse established by a UCP2-immunoreactive axon terminal in the arcuate nucleus. In F, note the abundance of UCP2-labeled mitochondria in an axon of the internal layer of the median eminence, which is the neuronal link between the magnocellular, paraventricular, and supraoptic nuclei and the posterior lobe of the pituitary. G, A large axon terminal containing UCP2-labeled mitochondria (arrows) in the external layer of the median eminence is in direct apposition to a portal capillary (cap), which provides the humoral link between the hypothalamus and the anterior pituitary.Asterisks indicate mitochondria with no UCP2 immunolabeling. Note that synaptic vesicles seem to be devoid of UCP2 labeling. Scale bar, 1 μm.
Fig. 5.
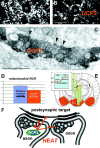
Brain UCP2 in axons predicts increased mitochondrial proton leak and heat production. Light (A, B) and electron (C) micrographs demonstrate the abundant expression of UCP2 in presynaptic axon terminals. Arrows on the light micrographs of_A_ and B point to UCP2-containing presynaptic terminals in the central amygdaloid nucleus that establish symmetrical synapses (C, arrowheads) on the postsynaptic target. D, The measurement of mitochondrial respiration in brain regions (left panel) where UCP2 is present (hypothalamus) and where no UCP2 was detected (thalamus/striatum) showed a lower mitochondrial phosphorylation level (lower RCR) in the hypothalamus that was caused exclusively by an increased proton leak of mitochondria in UCP2-containing regions.E, In agreement with this increased mitochondrial uncoupling activity, brain temperature in the UCP2-expressing hypothalamus was significantly higher than that of the thalamus (right panel) and the core body temperature.F, The presence of UCP2 in axon terminals together with the significant, positive correlation between UCP2 and mitochondrial uncoupling and local brain temperature suggests that heat produced presynaptically by UCP2 may have a direct influence on axonal temperature leading to the modulation of presynaptic and postsynaptic events.
Fig. 6.
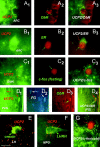
UCP2 in hypothalamic peptidergic circuits. Fluorescence double labeling using heterologous antisera revealed UCP2 immunoreactivity (left panels,green fluorescence) in paraventricular neurons producing CRF (top right panel,red fluorescence; arrows_point to the double-immunolabeled cells in_A1 and A2), in CRF-containing axon terminals in the external layer of the median eminence (B1,B2), in NPY-containing arcuate nucleus neurons (C1, C2), and in VP-expressing cells of the supraoptic nucleus (D1, D2).
Fig. 7.
Relationship between UCP2 and neuronal circuits involved in endocrine and metabolic regulation.A 1 –A3, Green fluorescent UCP2-immunoreactive axon terminals (A1, red arrowheads) in close proximity to an arcuate nucleus neuron expressing immunoreactivity for receptor of the peripheral metabolic hormone leptin (ObR;A2). The image of_A3_ was taken using filters sensitive for both red and green fluorescence._B1_–B3 , Green fluorescent, UCP2-immunoreactive perikaryonin the arcuate nucleus containing labeling for the peripheral sex hormone estradiol (ER; red fluorescence).C 1 –C3 , Green fluorescent UCP2-immunoreactive axon terminals (red arrowheads) in close proximity to a perikaryon of the dorsomedial hypothalamic nucleus (DMN) that was activated by fasting (red fluorescent, nuclear c-fos labeling). Fasting-induced c-fos expression was also detected in the lateral hypothalamus-perifornical region, arcuate nucleus, medial preoptic area, central amygdaloid nucleus, and the paraventricular thalamic nucleus.D1–D4 , Green fluorescent UCP2-producing parvicellular neurons of the paraventricular nucleus (PVN;D1, arrows) are neuroendocrine as revealed by retrograde tracing from the periphery using fluorogold (D2 , FG; FG labeling vas visualized using transillumination with ultraviolet light). The UCP2-expressing neuroendocrine cell located to the right(D3, D4,green arrows) also contains leptin receptors (ObR; D 3 , red fluorescence). E, Red fluorescent, UCP2-containing axon terminals (red arrows) in close proximity to a neuronal perikaryon (green arrowhead) in the lateral hypothalamus (LH) that expresses the appetite-inducing peptide orexin. F, Red fluorescent, UCP2-containing axon terminals (red arrows) in close proximity to a hypophyseotropic neuron that produce luteinizing hormone-releasing hormone (LHRH) in the medial preoptic region (MPO). G, Green fluorescent UCP2-immunoreactive axon terminals (red arrowheads) in close proximity to a perikaryon of MPO that was activated by cold exposure (red fluorescent, nuclear c-fos labeling). Other cold-activated neurons were distributed in the paraventricular nucleus, medial preoptic area, bed nucleus of the stria terminalis, central amygdaloid nucleus, and the paraventricular thalamic nucleus.
Similar articles
- Mitochondrial uncoupling protein 2 (UCP2) in the nonhuman primate brain and pituitary.
Diano S, Urbanski HF, Horvath B, Bechmann I, Kagiya A, Nemeth G, Naftolin F, Warden CH, Horvath TL. Diano S, et al. Endocrinology. 2000 Nov;141(11):4226-38. doi: 10.1210/endo.141.11.7740. Endocrinology. 2000. PMID: 11089557 - Energy gradients for VT-signal migration in the CNS: studies on melanocortin receptors, mitochondrial uncoupling proteins and food intake.
Agnati LF, Vergoni AV, Leo G, Genedani S, Franco R, Bertolini A, Fuxe K. Agnati LF, et al. J Endocrinol Invest. 2004;27(6 Suppl):23-34. J Endocrinol Invest. 2004. PMID: 15481801 - Uncoupling protein 2 in the brain: distribution and function.
Richard D, Clavel S, Huang Q, Sanchis D, Ricquier D. Richard D, et al. Biochem Soc Trans. 2001 Nov;29(Pt 6):812-7. doi: 10.1042/0300-5127:0290812. Biochem Soc Trans. 2001. PMID: 11709080 Review. - Distribution of the uncoupling protein 2 mRNA in the mouse brain.
Richard D, Rivest R, Huang Q, Bouillaud F, Sanchis D, Champigny O, Ricquier D. Richard D, et al. J Comp Neurol. 1998 Aug 10;397(4):549-60. J Comp Neurol. 1998. PMID: 9699915 - Brain distribution of UCP2 mRNA: in situ hybridization histochemistry studies.
Richard D, Huang Q, Sanchis D, Ricquier D. Richard D, et al. Int J Obes Relat Metab Disord. 1999 Jun;23 Suppl 6:S53-5. doi: 10.1038/sj.ijo.0800947. Int J Obes Relat Metab Disord. 1999. PMID: 10454125 Review.
Cited by
- Noncoupled Mitochondrial Respiration as Therapeutic Approach for the Treatment of Metabolic Diseases: Focus on Transgenic Animal Models.
Gureev AP, Alimova AA, Silachev DN, Plotnikov EY. Gureev AP, et al. Int J Mol Sci. 2023 Nov 18;24(22):16491. doi: 10.3390/ijms242216491. Int J Mol Sci. 2023. PMID: 38003681 Free PMC article. Review. - Using Intermittent Fasting as a Non-pharmacological Strategy to Alleviate Obesity-Induced Hypothalamic Molecular Pathway Disruption.
Oliveira LDC, Morais GP, Ropelle ER, de Moura LP, Cintra DE, Pauli JR, de Freitas EC, Rorato R, da Silva ASR. Oliveira LDC, et al. Front Nutr. 2022 Mar 30;9:858320. doi: 10.3389/fnut.2022.858320. eCollection 2022. Front Nutr. 2022. PMID: 35445066 Free PMC article. Review. - Uncoupling Proteins and Regulated Proton Leak in Mitochondria.
Ardalan A, Smith MD, Jelokhani-Niaraki M. Ardalan A, et al. Int J Mol Sci. 2022 Jan 28;23(3):1528. doi: 10.3390/ijms23031528. Int J Mol Sci. 2022. PMID: 35163451 Free PMC article. Review. - Mitochondrial Uncoupling Proteins (UCPs) as Key Modulators of ROS Homeostasis: A Crosstalk between Diabesity and Male Infertility?
Monteiro BS, Freire-Brito L, Carrageta DF, Oliveira PF, Alves MG. Monteiro BS, et al. Antioxidants (Basel). 2021 Oct 30;10(11):1746. doi: 10.3390/antiox10111746. Antioxidants (Basel). 2021. PMID: 34829617 Free PMC article. Review. - Infrared neural stimulation and inhibition using an implantable silicon photonic microdevice.
Horváth ÁC, Borbély S, Boros ÖC, Komáromi L, Koppa P, Barthó P, Fekete Z. Horváth ÁC, et al. Microsyst Nanoeng. 2020 Jun 1;6:44. doi: 10.1038/s41378-020-0153-3. eCollection 2020. Microsyst Nanoeng. 2020. PMID: 34567656 Free PMC article.
References
- Andersen P, Moser EI. Brain temperature and hippocampal function. Hippocampus. 1995;5:491–498. - PubMed
- Boss O, Muzzin P, Giacobino JP. The uncoupling proteins, a review. Eur J Endocrinol. 1998;139:1–9. - PubMed
- Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous