Selective discrimination learning impairments in mice expressing the human Huntington's disease mutation - PubMed (original) (raw)
Selective discrimination learning impairments in mice expressing the human Huntington's disease mutation
L A Lione et al. J Neurosci. 1999.
Abstract
Cognitive decline is apparent in the early stages of Huntington's disease and progressively worsens throughout the course of the disease. Expression of the human Huntington's disease mutation in mice (R6/2 line) causes a progressive neurological phenotype with motor symptoms resembling those seen in Huntington's disease. Here we describe the cognitive performance of R6/2 mice using four different tests (Morris water maze, visual cliff avoidance, two-choice swim tank, and T-maze). Behavioral testing was performed on R6/2 transgenic mice and their wild-type littermates between 3 and 14.5 weeks of age, using separate groups of mice for each test. R6/2 mice did not show an overt motor phenotype until approximately 8 weeks of age. However, between 3.5 and 8 weeks of age, R6/2 mice displayed progressive deterioration in specific aspects of learning in the Morris water maze, visual cliff, two-choice swim tank, and T-maze tasks. The age of onset and progression of the deficits in the individual tasks differed depending on the particular task demands. Thus, as seen in humans with Huntington's disease, R6/2 mice develop progressive learning impairments on cognitive tasks sensitive to frontostriatal and hippocampal function. We suggest that R6/2 mice provide not only a model for studying cognitive and motor changes in trinucleotide repeat disorders, but also a framework within which the functional efficacy of therapeutic strategies aimed at treating such diseases can be tested.
Figures
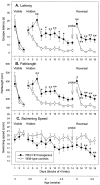
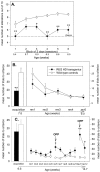
Fig. 1.
Impairment of Morris water maze learning in R6/2 mice. Escape latency (A), pathlength (B), and swimming speed (C) of control (n = 20) and R6/2 (_n_= 23) mice during acquisition of visible (days 1–3), hidden (days 4–14), and reversal (days 15–19) learning. R6/2 mice were unimpaired in swimming to a visible platform compared with controls (A–C). The latency (A) and pathlength (B) to escape to the hidden platform was impaired in R6/2 mice relative to control mice. Note that control and R6/2 mice did not differ in initial escape latency (A), pathlength (B), or swimming speed (C), indicating that R6/2 mice did not show nonspecific sensory impairment. R6/2 mice were impaired relative to controls in ability to learn reversal place learning of a hidden platform. There was no significant difference in swimming speed between R6/2 and control mice during the visible and hidden trials, but during reversal trials swimming speeds were significantly different between the two groups. Symbols indicate means ± SEM by mice of each group on each measure. _Asterisks_indicate significant differences between control and R6/2 mice (*p < 0.05; **p < 0.01).
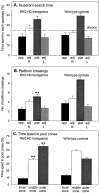
Fig. 2.
Impairment of probe trial performance in R6/2 mice. During the probe trials, R6/2 and control groups spent significantly >25% of their time in the platform quadrant, indicating that all mice had learned the platform location (A). The implications of A are refuted by the observation that R6/2 mice crossed what had been the exact location of the platform significantly less frequently than did controls (B) and furthermore that R6/2 mice did not show a preference for platform crossings over equivalent sites in the other quadrants. R6/2 mice spent significantly more time in the peripheral outer pool zone and significantly less time in the middle pool zone relative to control mice (C).Vertical bars indicate the SEM. _Asterisks_indicate significant preferences between measures (**p < 0.01).
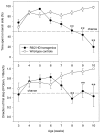
Fig. 3.
Deficient visual cliff avoidance in R6/2 mice. Separate groups of mice were tested between 3 and 10 weeks of age. Visual cliff avoidance of mice was measured using the percentage of time spent on the bench side of the visual cliff open field, as well as the direction of the first step outside of the start area (0 equates to open side step and 1 equates to bench side step). Control (n = 34) and R6/2 (n = 30) mice spent significantly more time on the bench side of the visual cliff box from 3–7 weeks of age (A). From 8 weeks of age, R6/2 mice spent significantly less time on the bench side relative to controls. A similar profile was seen for control and R6/2 mice for the direction of first step measure (B).Symbols indicate means ± SEM at each age.Asterisks indicate significant differences between control and R6/2 mice (**p < 0.01).
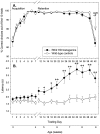
Fig. 4.
Normal visual discrimination learning and memory independent of swimming dysfunction in R6/2 mice. Control (n = 17) and R6/2 (n = 14) mice were tested between 3 and 9 weeks of age. Control and R6/2 mice displayed a comparable degree of acquisition of a two-choice swim tank visual discrimination task at 3–4 weeks of age, and both groups maintained performance criterion until 8.5 weeks of age (A). On removal of the light stimulus at 9 weeks of age, performance declined to chance levels for both groups. R6/2 mice displayed swimming impairments from 5 weeks of age, which progressively worsened, as compared with controls (B). Motoric dysfunction failed to impair performance of R6/2 mice in the two-choice swim tank task.Vertical bars indicate means ± SEM of each measure across all trials at each age. Asterisks indicate significant differences between control and R6/2 mice (*p < 0.05; **p < 0.01).
Fig. 5.
Selective deficits in visual and reversal discrimination learning in R6/2 mice. Separate groups of control (n = 10–18) and R6/2 (n = 12–21) mice were tested between 3 and 10 weeks of age. Control and R6/2 mice displayed a comparable degree of acquisition of a two-choice swim tank visual discrimination task at 3–4 (A) and 5–6 (B) weeks of age, however R6/2 mice displayed significantly slower learning than controls at 7–8 weeks of age (C), and by 10–11 weeks of age (D) acquisition was severely impaired in R6/2 mice. In contrast, acquisition of reversal discrimination was impaired in R6/2 mice from 6.5 weeks of age (E–H).Symbols indicate means ± SEM by mice of each group at each age on both measures. Asterisks indicate significant differences between control and R6/2 mice (*p < 0.05; **p < 0.01).
Fig. 6.
Alternation learning impairments in R6/2 mice. R6/2 mice (n = 15) were impaired in T-maze alternation (A) but unimpaired in a simple T-maze black–white visual discrimination test (B), compared with controls (n = 13). Whereas control mice adopted spatial strategies, R6/2 mice used nonspatial strategies (C). Symbols indicate means ± SEM by mice of each group on each measure. _Asterisks_indicate significant differences between control and R6/2 mice (*p < 0.05; **p < 0.01).
Fig. 7.
Schematic representation of the earliest age of onset of impairment in spatial, visual, reversal, and alternation learning in R6/2 mice. R6/2 mice showed spatial learning impairments in the Morris water maze from 3.5 weeks (A), alternation learning impairments in the T-maze from 5 weeks (B), and reversal learning impairments in the two-choice swim tank from 6.5 weeks (C). Visual discriminative learning impairments were first observed from 7–8 weeks (D, E), and deterioration in retention of a previously learned visual task was not seen until 8.5 weeks (F). Filled bars indicate onset of impairment.
Similar articles
- Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation.
Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Carter RJ, et al. J Neurosci. 1999 Apr 15;19(8):3248-57. doi: 10.1523/JNEUROSCI.19-08-03248.1999. J Neurosci. 1999. PMID: 10191337 Free PMC article. - Evaluation of R6/2 HD transgenic mice for therapeutic studies in Huntington's disease: behavioral testing and impact of diabetes mellitus.
Lüesse HG, Schiefer J, Spruenken A, Puls C, Block F, Kosinski CM. Lüesse HG, et al. Behav Brain Res. 2001 Nov 29;126(1-2):185-95. doi: 10.1016/s0166-4328(01)00261-3. Behav Brain Res. 2001. PMID: 11704263 - A comparison of discrimination learning in touchscreen and 2-choice swim tank using an allelic series of Huntington's disease mice.
Glynn D, Skillings EA, Morton AJ. Glynn D, et al. J Neurosci Methods. 2016 May 30;265:56-71. doi: 10.1016/j.jneumeth.2015.07.016. Epub 2015 Jul 26. J Neurosci Methods. 2016. PMID: 26219658 - Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington's disease mutation.
Murphy KP, Carter RJ, Lione LA, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Murphy KP, et al. J Neurosci. 2000 Jul 1;20(13):5115-23. doi: 10.1523/JNEUROSCI.20-13-05115.2000. J Neurosci. 2000. PMID: 10864968 Free PMC article. - Progressive imbalance in the interaction between spatial and procedural memory systems in the R6/2 mouse model of Huntington's disease.
Ciamei A, Morton AJ. Ciamei A, et al. Neurobiol Learn Mem. 2009 Oct;92(3):417-28. doi: 10.1016/j.nlm.2009.06.002. Epub 2009 Jun 12. Neurobiol Learn Mem. 2009. PMID: 19524696
Cited by
- Oral administration of the pimelic diphenylamide HDAC inhibitor HDACi 4b is unsuitable for chronic inhibition of HDAC activity in the CNS in vivo.
Beconi M, Aziz O, Matthews K, Moumné L, O'Connell C, Yates D, Clifton S, Pett H, Vann J, Crowley L, Haughan AF, Smith DL, Woodman B, Bates GP, Brookfield F, Bürli RW, McAllister G, Dominguez C, Munoz-Sanjuan I, Beaumont V. Beconi M, et al. PLoS One. 2012;7(9):e44498. doi: 10.1371/journal.pone.0044498. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22973455 Free PMC article. - Pathological role of apoptosis signal-regulating kinase 1 in human diseases and its potential as a therapeutic target for cognitive disorders.
Cheon SY, Cho KJ. Cheon SY, et al. J Mol Med (Berl). 2019 Feb;97(2):153-161. doi: 10.1007/s00109-018-01739-9. Epub 2019 Jan 7. J Mol Med (Berl). 2019. PMID: 30617854 Review. - The corticostriatal pathway in Huntington's disease.
Cepeda C, Wu N, André VM, Cummings DM, Levine MS. Cepeda C, et al. Prog Neurobiol. 2007 Apr;81(5-6):253-71. doi: 10.1016/j.pneurobio.2006.11.001. Epub 2006 Dec 13. Prog Neurobiol. 2007. PMID: 17169479 Free PMC article. Review. - Asialoerythropoietin is not effective in the R6/2 line of Huntington's disease mice.
Gil JM, Leist M, Popovic N, Brundin P, Petersén A. Gil JM, et al. BMC Neurosci. 2004 May 10;5:17. doi: 10.1186/1471-2202-5-17. BMC Neurosci. 2004. PMID: 15134587 Free PMC article. - Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function.
Raymond LA, André VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Raymond LA, et al. Neuroscience. 2011 Dec 15;198:252-73. doi: 10.1016/j.neuroscience.2011.08.052. Epub 2011 Aug 27. Neuroscience. 2011. PMID: 21907762 Free PMC article. Review.
References
- Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986;321:168–171. - PubMed
- Borlongan CV, Koutouzis TK, Freeman TB, Cahill DW, Sanberg PR. Behavioral pathology induced by repeated systemic injections of 3-nitropropionic acid mimics the motoric symptoms of Huntington's disease. Brain Res. 1995;697:254–257. - PubMed
- Bossi SR, Simpson JR, Isacson O. Age dependence of striatal neuronal death caused by mitochondrial dysfunction. NeuroReport. 1993;4:73–76. - PubMed
- Brouillet E, Jenkins BG, Hyman BT, Ferrante RJ, Kowall NW, Srivastava R, Roy DS, Rosen BR, Beal MF. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J Neurochem. 1993;60:356–359. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical