Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy - PubMed (original) (raw)
Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy
T Sullivan et al. J Cell Biol. 1999.
Abstract
The nuclear lamina is a protein meshwork lining the nucleoplasmic face of the inner nuclear membrane and represents an important determinant of interphase nuclear architecture. Its major components are the A- and B-type lamins. Whereas B-type lamins are found in all mammalian cells, A-type lamin expression is developmentally regulated. In the mouse, A-type lamins do not appear until midway through embryonic development, suggesting that these proteins may be involved in the regulation of terminal differentiation. Here we show that mice lacking A-type lamins develop to term with no overt abnormalities. However, their postnatal growth is severely retarded and is characterized by the appearance of muscular dystrophy. This phenotype is associated with ultrastructural perturbations to the nuclear envelope. These include the mislocalization of emerin, an inner nuclear membrane protein, defects in which are implicated in Emery-Dreifuss muscular dystrophy (EDMD), one of the three major X-linked dystrophies. Mice lacking the A-type lamins exhibit tissue-specific alterations to their nuclear envelope integrity and emerin distribution. In skeletal and cardiac muscles, this is manifest as a dystrophic condition related to EDMD.
Figures
Figure 1
Targeting of the Lmna gene. (a) Structure of the mouse Lmna gene, together with the targeting vector and homologous recombinant containing the PgkNeo cassette. (b) Southern analysis of the representative genotypes from Lmna heterozygote crosses. (c) Northern analysis from wild-type and Lmna null fibroblasts showing loss of full length forms of lamin A and C mRNAs. These are replaced by truncated transcripts at levels 10 fold below those of wild type. (d) Western analysis, with the XB10 antibody (to the central domain of lamin A/C) of nuclear extracts from MEFs of all three genotypes showing that lamin A and C proteins were undetectable in the −/− fibroblasts, whereas lamin B levels were unaffected. P19 EC cells were used as a negative control. (e) Western analysis, using the 1E4 antibody (against the lamin A/C amino-terminal region) of liver nuclei and nuclear envelopes showing, as in the MEFs' absence of the lamin A proteins.
Figure 2
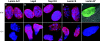
Immunohistochemical analysis of wild-type and Lmna null MEFs. The nuclei in all experiments were labeled with DAPI; in a–d, DAPI and immunofluorescence images have been superimposed. (a) Lamins A and C were detected with the XB10 mAb. (b) Nuclear lamina–associated protein, LAP2. Note the uniform distribution of this protein in wild-type nuclear envelopes, but its loss from one pole of the irregularly shaped nuclei in the Lmna null cells. (c) Nuclear pore complex Nup153. As with LAP2, there is loss from one pole of the nucleus, indicating exclusion of NPCs from this region. (d and e) Lamin B. Exclusion of this protein from one pole of the nucleus is clearly seen in the Lmna null cells. In e, the immunofluorescence image showing lamin B distribution (Lamin B*) has been superimposed on the Nomarski image of the same cell. Note that the two panels in e show complementary images of the same Lmna null cell. The lower panel shows DAPI labeling only.
Figure 3
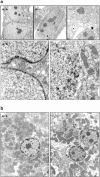
Electron microscopic analysis of MEFs (a) and hepatocytes (b). (a) In the wild-type and heterozygous MEF nuclei, a largely continuous layer of heterochromatin in contact with the inner face of the nuclear envelope is visible. In the Lmna null nuclei, there are obvious discontinuities in this layer (orange box) that are juxtaposed to distensions of the nuclear membranes. (b) Similar discontinuities can be seen in the liver nuclei (arrow).
Figure 4
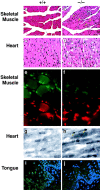
Histological and immunohistochemical analysis of tissues from Lmna null mice. (a) Wild-type perivertebral muscle showing the peripheral localization of the nuclei in the muscle fibers. (b) Perivertebral muscles from Lmna null mice showing an increase in nuclear number with many centrally located within the muscle fibers (arrows). (c) Wild-type ventricular cardiac muscle. (d) Ventricular muscle from a Lmna null mouse. (e and f) Emerin (upper panels) localization in the skeletal nuclei of wild-type (e) and lamin −/− (f) mice. In the −/− muscle, the signal intensity of emerin in the NE is weaker than in the +/+ nuclei. Anti–lamin B labeling is unchanged (lower panels rhodamine label). (g and h) Emerin staining in the cardiac nuclei of wild-type (g) and lmna −/− (h) mice. Note the polar distribution (arrows) of emerin in the −/− cardiac muscle NEs. (i and j) Emerin staining in the tongue epithelium. In wild-type epithelium, emerin localization to the NE is readily detectable (i), whereas in the lamin null mice it is lost (j). Nuclei i and j are costained with DAPI.
Figure 5
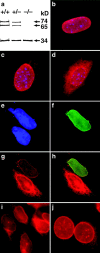
(a) Western blot analysis of emerin (34 kD) and lamins A and C (74 and 65 kD, respectively) in +/+, +/−, and −/− Lmna MEFs showing no difference in emerin levels between different genotypes. (b) In wild-type cells, emerin is localized exclusively to the nuclear envelope. (c) In the heterozygotes, some emerin localization can be detected in the cytoplasm. (d) In the lamin A/C −/− cells, emerin is largely cytoplasmic although some localization to the nuclear envelope is still apparent. Expression of human lamin A in Lmna null MEFs results in relocalization of emerin to the nuclear envelope. (e) DAPI staining revealing the nuclei of two MEFs. (f) The upper MEF expresses human lamin A localized exclusively to the nuclear envelope. (g) Both cells express emerin but in the lamin A null cell, emerin is localized predominantly in the cytoplasmic compartment, whereas in the lamin A positive MEF, emerin is localized almost entirely to the nuclear envelope. (h) Merged image showing colocalization of emerin and human lamin A in the nuclear envelope. (i and j) Emerin distribution in P19EC cells (i) and (j) their differentiated derivatives P19MES that express A-type lamins. Both cell types exhibit NE-associated emerin.
Similar articles
- Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy.
Raharjo WH, Enarson P, Sullivan T, Stewart CL, Burke B. Raharjo WH, et al. J Cell Sci. 2001 Dec;114(Pt 24):4447-57. doi: 10.1242/jcs.114.24.4447. J Cell Sci. 2001. PMID: 11792810 - The A-type lamins: nuclear structural proteins as a focus for muscular dystrophy and cardiovascular diseases.
Mounkes LC, Burke B, Stewart CL. Mounkes LC, et al. Trends Cardiovasc Med. 2001 Oct;11(7):280-5. doi: 10.1016/s1050-1738(01)00126-8. Trends Cardiovasc Med. 2001. PMID: 11709282 Review. - Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity.
Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM. Zhang Q, et al. Hum Mol Genet. 2007 Dec 1;16(23):2816-33. doi: 10.1093/hmg/ddm238. Epub 2007 Aug 29. Hum Mol Genet. 2007. PMID: 17761684 - Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration.
Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, Sartorelli V, Seo J, Pegoraro E, Angelini C, Shneiderman B, Escolar D, Chen YW, Winokur ST, Pachman LM, Fan C, Mandler R, Nevo Y, Gordon E, Zhu Y, Dong Y, Wang Y, Hoffman EP. Bakay M, et al. Brain. 2006 Apr;129(Pt 4):996-1013. doi: 10.1093/brain/awl023. Epub 2006 Feb 14. Brain. 2006. PMID: 16478798 - Nuclear envelope defects in muscular dystrophy.
Roux KJ, Burke B. Roux KJ, et al. Biochim Biophys Acta. 2007 Feb;1772(2):118-27. doi: 10.1016/j.bbadis.2006.06.001. Epub 2006 Jun 7. Biochim Biophys Acta. 2007. PMID: 16904876 Review.
Cited by
- SIRT1 Ameliorates Lamin A/C Deficiency-Induced Cardiac Dysfunction by Promoting Mitochondrial Bioenergetics.
Du Z, Zhou Y, Li Q, Xie Y, Zhu T, Qiao J, Zhang R, Bao Y, Wang L, Xie Y, Quan J, Lin M, Zhang N, Jin Q, Liang W, Wu L, Yin T, Xie Y. Du Z, et al. JACC Basic Transl Sci. 2024 Jul 31;9(10):1211-1230. doi: 10.1016/j.jacbts.2024.05.011. eCollection 2024 Oct. JACC Basic Transl Sci. 2024. PMID: 39534638 Free PMC article. - Causes and consequences of DNA double-stranded breaks in cardiovascular disease.
Marian AJ. Marian AJ. Mol Cell Biochem. 2024 Oct 15. doi: 10.1007/s11010-024-05131-9. Online ahead of print. Mol Cell Biochem. 2024. PMID: 39404936 Review. - Tail-anchored membrane protein SLMAP3 is essential for targeting centrosomal proteins to the nuclear envelope in skeletal myogenesis.
Dias AP, Rehmani T, Salih M, Tuana B. Dias AP, et al. Open Biol. 2024 Oct;14(10):240094. doi: 10.1098/rsob.240094. Epub 2024 Oct 9. Open Biol. 2024. PMID: 39378988 Free PMC article. - Emerin mislocalization during chromatin bridge resolution can drive prostate cancer cell invasiveness in a collagen-rich microenvironment.
Popęda M, Kowalski K, Wenta T, Beznoussenko GV, Rychłowski M, Mironov A, Lavagnino Z, Barozzi S, Richert J, Bertolio R, Myszczyński K, Szade J, Bieńkowski M, Miszewski K, Matuszewski M, Żaczek AJ, Braga L, Del Sal G, Bednarz-Knoll N, Maiuri P, Nastały P. Popęda M, et al. Exp Mol Med. 2024 Sep;56(9):2016-2032. doi: 10.1038/s12276-024-01308-w. Epub 2024 Sep 2. Exp Mol Med. 2024. PMID: 39218980 Free PMC article. - The Pathogenic Mechanisms of and Novel Therapies for Lamin A/C-Related Dilated Cardiomyopathy Based on Patient-Specific Pluripotent Stem Cell Platforms and Animal Models.
Wu XY, Lee YK, Lau YM, Au KW, Tse YL, Ng KM, Wong CK, Tse HF. Wu XY, et al. Pharmaceuticals (Basel). 2024 Aug 5;17(8):1030. doi: 10.3390/ph17081030. Pharmaceuticals (Basel). 2024. PMID: 39204134 Free PMC article. Review.
References
- Bione S., Maestrini E., Rivella S., Mancini M., Regis S., Romeo G., Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 1994;8:323–327. - PubMed
- Bodoor K., Shaihk S.A., Salina D., Raharjo W.H., Bastos R., Lohka M.J., Burke B. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J. Cell Sci. 1999;112:2253–2264. - PubMed
- Bonne G., Di Barletta M.R., Varnous S., Becane H.M., Hammouda E.H., Merlini L., Muntoni F., Greenberg C.R., Gary F., Urtizberea J.A. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 1999;21:285–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases