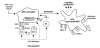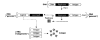DNA and RNA-based vaccines: principles, progress and prospects - PubMed (original) (raw)
Review
DNA and RNA-based vaccines: principles, progress and prospects
W W Leitner et al. Vaccine. 1999.
Abstract
DNA vaccines were introduced less than a decade ago but have already been applied to a wide range of infectious and malignant diseases. Here we review the current understanding of the mechanisms underlying the activities of these new vaccines. We focus on recent strategies designed to enhance their function including the use of immunostimulatory (CpG) sequences, dendritic cells (DC), co-stimulatory molecules and cytokine- and chemokine-adjuvants. Although genetic vaccines have been significantly improved, they may not be sufficiently immunogenic for the therapeutic vaccination of patients with infectious diseases or cancer in clinical trials. One promising approach aimed at dramatically increasing the immunogenicity of genetic vaccines involves making them 'self-replicating'. This can be accomplished by using a gene encoding RNA replicase, a polyprotein derived from alphaviruses, such as Sindbis virus. Replicase-containing RNA vectors are significantly more immunogenic than conventional plasmids, immunizing mice at doses as low as 0.1 microg of nucleic acid injected once intramuscularly. Cells transfected with 'self-replicating' vectors briefly produce large amounts of antigen before undergoing apoptotic death. This death is a likely result of requisite double-stranded (ds) RNA intermediates, which also have been shown to super-activate DC. Thus, the enhanced immunogenicity of 'self-replicating' genetic vaccines may be a result of the production of pro-inflammatory dsRNA, which mimics an RNA-virus infection of host cells.
Figures
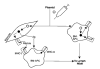
Fig. 1. Transfection of host cells with plasmid DNA
Plasmid (◯) is actively or passively taken up by host cells. Antigen (ν) produced by transfected myocytes can be taken up by bone marrow (BM)-derived APCs. Alternatively, BM-APC can be transfected directly. Antigen-bearing APC then can process and present peptides complexed with MHC-molecules to the immune system after migrating to lymphoid tissue.
Fig. 2. ‘Super-activation’ of APC after vaccination with genetic vaccines
APC are initially stimulated in the injected tissue by either needle or gene-gun delivery of plasmid. Immunostimulatory sequences (ISS) stimulate host APCs, T cells and NK cells. APC interact with T cells through both TCR/MHC and CD40/CD40L, triggering the upregulation of B7 expression on the APC. ISS also polyclonally stimulate B-cells and induce the release of IL-6 and IL-12, further promoting a Th1 response.
Fig. 3. Self-replicating genetic vaccines
The first product of the self-replicating RNA is a four-subunit-replicase which uses the (+) strand RNA as a template to make (−) strand RNA and more copies of full length (+) strand ‘genomic’ RNA and (+) strand ‘subgenomic’ mRNA for the encoded antigen. Due to the high number of RNA-copies, the main product of the transfected cells becomes the encoded antigen. The host cell eventually undergoes apoptosis.
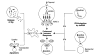
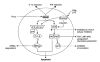
Fig. 4. Potential factors contributing to the high immunogenicity of self-replicating genetic vaccines
(Starting in the upper centre and moving clockwise): Accumulation of antigen in the transfected cell can result in highly efficient MHC-I-loading. A number of ‘danger signals’ may be generated such as interferon production and interferon release from infected cells resulting from the presence of dsRNA. Interferon may also be produced by bystander cells in response to dsRNA released from dead and lysed transfected cells. Heat shock proteins (HSP) have also been shown to be produced in response to the presence of dsRNA in the cells. Ingestion of antigen-loaded apoptotic cells by APCs can also result in the elicitation of powerful immune responses. Finally, the local release of large amounts of antigen at the site of injection by transfected cells may be fed into resident APC.
Fig. 5. dsRNA might be a central element in the immunogenicity of replicase-based genetic vaccines
dsRNA is produced in cells infected with RNA-viruses, but is also expected to be an intermediate in the replicase-mediated duplication of mRNA after delivery of ‘self-replicating’ genetic vaccines. dsRNA is a potent inducer of interferon, which in turn induces the expression of PKR and 2-5A synthetase. These enzymes are activated by dsRNA and mediate at least some of the cellular effects associated with viral infection, including apoptotic cell death, aimed at preventing viral spread. The ‘danger signals’ resulting from the activation of these two enzymes could account for the increased immunogenicity of replicase-based genetic vaccines, providing an adjuvant-effect.
Similar articles
- Cancer therapy using a self-replicating RNA vaccine.
Ying H, Zaks TZ, Wang RF, Irvine KR, Kammula US, Marincola FM, Leitner WW, Restifo NP. Ying H, et al. Nat Med. 1999 Jul;5(7):823-7. doi: 10.1038/10548. Nat Med. 1999. PMID: 10395329 Free PMC article. - Type I Interferons are essential for the efficacy of replicase-based DNA vaccines.
Leitner WW, Bergmann-Leitner ES, Hwang LN, Restifo NP. Leitner WW, et al. Vaccine. 2006 Jun 12;24(24):5110-8. doi: 10.1016/j.vaccine.2006.04.059. Epub 2006 May 6. Vaccine. 2006. PMID: 16725231 Free PMC article. - Nucleic acid for the treatment of cancer: genetic vaccines and DNA adjuvants.
Leitner WW, Hammerl P, Thalhamer J. Leitner WW, et al. Curr Pharm Des. 2001 Nov;7(16):1641-67. doi: 10.2174/1381612013397249. Curr Pharm Des. 2001. PMID: 11562304 Review. - Enhancement of sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments.
Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Ling M, Slater LA, Roden RB, Wu TC. Cheng WF, et al. Hum Gene Ther. 2001 Feb 10;12(3):235-52. doi: 10.1089/10430340150218387. Hum Gene Ther. 2001. PMID: 11177561 - Self-replicating alphavirus RNA vaccines.
Ljungberg K, Liljeström P. Ljungberg K, et al. Expert Rev Vaccines. 2015 Feb;14(2):177-94. doi: 10.1586/14760584.2015.965690. Epub 2014 Oct 1. Expert Rev Vaccines. 2015. PMID: 25269775 Review.
Cited by
- Self Replicating Gene Vaccine Carrying P1-2A Gene of FMDV Serotype O and its Effects on the Immune Responses of Cattle.
Nagarajan G, Ravikumar P, Ashok Kumar C, Reddy GR, Dechamma HJ, Suryanarayana VV. Nagarajan G, et al. Indian J Virol. 2011 Jun;22(1):50-8. doi: 10.1007/s13337-011-0032-5. Epub 2011 May 10. Indian J Virol. 2011. PMID: 23637502 Free PMC article. - CpG motif acts as a 'danger signal' and provides a T helper type 1-biased microenvironment for DNA vaccination.
Liu L, Zhou X, Liu H, Xiang L, Yuan Z. Liu L, et al. Immunology. 2005 Jun;115(2):223-30. doi: 10.1111/j.1365-2567.2005.02150.x. Immunology. 2005. PMID: 15885128 Free PMC article. - Myth, menace or medical blessing? The clinical potential and the problems of genetic vaccines.
Leitner WW. Leitner WW. EMBO Rep. 2001 Mar;2(3):168-70. doi: 10.1093/embo-reports/kve054. EMBO Rep. 2001. PMID: 11266352 Free PMC article. No abstract available. - Current Status and Challenges of Vaccination Therapy for Glioblastoma.
Hosseinalizadeh H, Rahmati M, Ebrahimi A, O'Connor RS. Hosseinalizadeh H, et al. Mol Cancer Ther. 2023 Apr 3;22(4):435-446. doi: 10.1158/1535-7163.MCT-22-0503. Mol Cancer Ther. 2023. PMID: 36779991 Free PMC article. - Towards effective COVID‑19 vaccines: Updates, perspectives and challenges (Review).
Calina D, Docea AO, Petrakis D, Egorov AM, Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F, Vinceti M, Spandidos DA, Tsatsakis A. Calina D, et al. Int J Mol Med. 2020 Jul;46(1):3-16. doi: 10.3892/ijmm.2020.4596. Epub 2020 May 6. Int J Mol Med. 2020. PMID: 32377694 Free PMC article. Review.
References
- Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. - PubMed
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–4. - PubMed
- Mor G. Plasmid DNA: a new era in vaccinology. Biochem Pharmacol. 1998;55:1151–3. - PubMed
- Whalen RG. DNA vaccines, cyberspace and self-help programs. Intervirology. 1996;39:120–5. - PubMed
- Wang B, Godillot AP, Madaio MP, Weiner DB, Williams WV. Vaccination against pathogenic cells by DNA inoculation. Curr Top Microbiol Immunol. 1998;226:21–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources