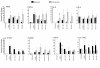Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions - PubMed (original) (raw)
Clinical Trial
. 1999 Dec;104(11):1527-37.
doi: 10.1172/JCI6910.
Affiliations
- PMID: 10587516
- PMCID: PMC409858
- DOI: 10.1172/JCI6910
Clinical Trial
Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions
W L Trepicchio et al. J Clin Invest. 1999 Dec.
Erratum in
- J Clin Invest 2000 Feb;105(3):396
Abstract
Psoriasis is a chronic inflammatory skin disease in which epidermal hyperplasia results from skin infiltration by type I T lymphocytes and release of associated cytokines. A multifunctional cytokine, rhIL-11, modulates macrophage and type I T-lymphocyte function in cell culture and shows anti-inflammatory activity in animal models. We are testing subcutaneous delivery of rhIL-11 to patients with psoriasis in a phase 1 open-label dose-escalation clinical trial. Tissue was obtained from lesional and uninvolved skin before and during treatment with rhIL-11 and was examined by histology/immunohistochemistry and quantitative RT-PCR. Expression of over 35 genes was examined in all patients, and multiple genetic markers of psoriasis were identified. Expression of numerous proinflammatory genes was elevated in psoriatic tissue compared with nonlesional skin. Seven of 12 patients responded well to rhIL-11 treatment. Amelioration of disease by rhIL-11, as shown by reduced keratinocyte proliferation and cutaneous inflammation, was associated with decreased expression of products of disease-related genes, including K16, iNOS, IFN-gamma, IL-8, IL-12, TNF-alpha, IL-1beta, and CD8, and with increased expression of endogenous IL-11. We believe that this is the first study in humans to indicate that type I cytokines can be selectively suppressed by an exogenous immune-modifying therapy. The study highlights the utility of pharmacogenomic monitoring to track patient responsiveness and to elucidate anti-inflammatory mechanisms.
Figures
Figure 1
Photographs of psoriatic plaques undergoing resolution in 2 patients (A and B) before and after 8 weeks of treatment with rhIL-11.
Figure 2
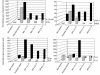
Six-millimeter punch biopsies were obtained from lesional and nonlesional skin of a patient before treatment with rhIL-11 (pretreatment) and at weeks 1, 4, and 8 following daily treatment with 2.5 mg/kg of rhIL-11. Biopsies were equally divided for immunohistochemical analysis and RNA preparation. Frozen biopsies from pretreatment, week 4, and week 8 were sectioned and stained with antibodies to Ki67+, K16, CD3, CD8, and ICAM-1. Enlargements of ICAM-1 staining (boxes) show elimination of its production by epidermal keratinocytes during IL-11 administration (large arrows).
Figure 3
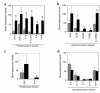
RNA was prepared from nonlesional and lesional skin of 6 untreated psoriasis patients. The mRNA was amplified for the indicated genes by quantitative RT-PCR. Levels of RNA were normalized to HARP. Average expression levels of nonlesional and lesional skin for all 6 patients are presented ± SEM. *Statistically significant differences (P < 0.05) between nonlesional and lesional skin; filled box, lesion; shaded box, nonlesion.
Figure 4
Six-millimeter punch biopsies were obtained from lesional and nonlesional skin of a responding patient before treatment with rhIL-11 (pretreatment) and at weeks 1, 4, and 8 following daily treatment with 2.5 mg/kg of rhIL-11. Biopsies were equally divided for immunohistochemical analysis and RNA preparation. RNA was prepared from nonlesional skin and lesional skin from pretreatment, week-1, week-4, and week-8 biopsies. The mRNA for IL-12 p40, IFN-γ, TNF-α, iNOS, CD8, IL-8, IL-4, and K16 were amplified by quantitative RT-PCR. Levels of mRNA were normalized to HARP to control for variations in starting RNA amounts.
Figure 5
RNA was prepared from nonlesional skin biopsies and lesional skin biopsies of 12 patients before treatment (pretreatment) and at week 1, week 4, and week 8 following daily treatment with 2.5 or 5.0 mg/kg of rhIL-11. Quantitative RT-PCR was performed on individual samples for the indicated genes. Gene expression levels were normalized to HARP. Levels of gene expression observed in the nonlesional skin of each patient were arbitrarily set to equal 1, and the fold change in expression in lesional skin before and after treatment with rhIL-11 over nonlesional skin was calculated. Average fold change for rhIL-11–responding (n = 7) and rhIL-11–nonresponding (n = 5) patients was calculated. Data are presented as the average fold change over nonlesional skin ± SEM. *Statistically significant differences between pretreatment and rhIL-11 treatment (P < 0.05); #statistically significant differences between responder and nonresponder pretreatment lesions (P < 0.05).
Similar articles
- Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation.
Reich K, Garbe C, Blaschke V, Maurer C, Middel P, Westphal G, Lippert U, Neumann C. Reich K, et al. J Invest Dermatol. 2001 Feb;116(2):319-29. doi: 10.1046/j.1523-1747.2001.01248.x. J Invest Dermatol. 2001. PMID: 11180010 Clinical Trial. - Proinflammatory cytokine production in HaCaT cells treated by eosin: implications for the topical treatment of psoriasis.
Zampetti A, Mastrofrancesco A, Flori E, Maresca V, Picardo M, Amerio P, Feliciani C. Zampetti A, et al. Int J Immunopathol Pharmacol. 2009 Oct-Dec;22(4):1067-75. doi: 10.1177/039463200902200423. Int J Immunopathol Pharmacol. 2009. PMID: 20074471 - Hematopoietic, immunomodulatory and epithelial effects of interleukin-11.
Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Schwertschlag US, et al. Leukemia. 1999 Sep;13(9):1307-15. doi: 10.1038/sj.leu.2401514. Leukemia. 1999. PMID: 10482979 Review. - Activation of mast cells mediates inflammatory response in psoriasis: Potential new therapeutic approach with IL-37.
Conti P, Gallenga CE, Ronconi G, Caraffa A, Kritas SK. Conti P, et al. Dermatol Ther. 2019 Jul;32(4):e12943. doi: 10.1111/dth.12943. Epub 2019 May 3. Dermatol Ther. 2019. PMID: 31012218 Review.
Cited by
- Ustekinumab in the therapy of chronic plaque psoriasis.
O'Neill JL, Kalb RE. O'Neill JL, et al. Biologics. 2009;3:159-68. Epub 2009 Jul 13. Biologics. 2009. PMID: 19707405 Free PMC article. - Fisetin, a 3,7,3',4'-Tetrahydroxyflavone Inhibits the PI3K/Akt/mTOR and MAPK Pathways and Ameliorates Psoriasis Pathology in 2D and 3D Organotypic Human Inflammatory Skin Models.
Chamcheu JC, Esnault S, Adhami VM, Noll AL, Banang-Mbeumi S, Roy T, Singh SS, Huang S, Kousoulas KG, Mukhtar H. Chamcheu JC, et al. Cells. 2019 Sep 15;8(9):1089. doi: 10.3390/cells8091089. Cells. 2019. PMID: 31540162 Free PMC article. - The Immunology of Psoriasis-Current Concepts in Pathogenesis.
Sieminska I, Pieniawska M, Grzywa TM. Sieminska I, et al. Clin Rev Allergy Immunol. 2024 Apr;66(2):164-191. doi: 10.1007/s12016-024-08991-7. Epub 2024 Apr 20. Clin Rev Allergy Immunol. 2024. PMID: 38642273 Free PMC article. Review. - Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti-CD6 itolizumab.
Aira LE, López-Requena A, Fuentes D, Sánchez L, Pérez T, Urquiza A, Bautista H, Falcón L, Hernández P, Mazorra Z. Aira LE, et al. MAbs. 2014 May-Jun;6(3):783-93. doi: 10.4161/mabs.28376. Epub 2014 Mar 4. MAbs. 2014. PMID: 24594862 Free PMC article. Clinical Trial. - Susceptibility-associated genetic variation at IL12B enhances Th1 polarization in psoriasis.
Johnston A, Xing X, Swindell WR, Kochkodan J, Riblett M, Nair RP, Stuart PE, Ding J, Voorhees JJ, Elder JT, Gudjonsson JE. Johnston A, et al. Hum Mol Genet. 2013 May 1;22(9):1807-15. doi: 10.1093/hmg/ddt034. Epub 2013 Jan 31. Hum Mol Genet. 2013. PMID: 23376980 Free PMC article.
References
- Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med. 1995;332:581–588. - PubMed
- Weinstein, G.D., and Kreuger, J.G. 1994. Overview of psoriasis. In Therapy of moderate to severe psoriasis. G.D. Weinstein and A.B. Gottlieb, editors. National Psoriasis Foundation. Portland, OR. 1–22.
- Gottlieb AB, et al. Studies of the effect of cyclosporine in psoriasis in vivo: combined effects on activated T lymphocytes and epidermal regenerative maturation. J Invest Dermatol. 1992;98:302–309. - PubMed
- Gottlieb SL, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune but not keratinocyte pathogenic basis. Nat Med. 1995;1:442–447. - PubMed
- Bachelez H, et al. Treatment of recalcitrant plaque psoriasis with a humanized non-depleting antibody to CD4. J Autoimmun. 1998;11:53–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-39214/AI/NIAID NIH HHS/United States
- GM-42461/GM/NIGMS NIH HHS/United States
- M01 RR000102/RR/NCRR NIH HHS/United States
- MO1-RR-00102/RR/NCRR NIH HHS/United States
- R01 GM042461/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials