Espin contains an additional actin-binding site in its N terminus and is a major actin-bundling protein of the Sertoli cell-spermatid ectoplasmic specialization junctional plaque - PubMed (original) (raw)
Espin contains an additional actin-binding site in its N terminus and is a major actin-bundling protein of the Sertoli cell-spermatid ectoplasmic specialization junctional plaque
B Chen et al. Mol Biol Cell. 1999 Dec.
Free PMC article
Abstract
The espins are actin-binding and -bundling proteins localized to parallel actin bundles. The 837-amino-acid "espin" of Sertoli cell-spermatid junctions (ectoplasmic specializations) and the 253-amino-acid "small espin" of brush border microvilli are splice isoforms that share a C-terminal 116-amino-acid actin-bundling module but contain different N termini. To investigate the roles of espin and its extended N terminus, we examined the actin-binding and -bundling properties of espin constructs and the stoichiometry and developmental accumulation of espin within the ectoplasmic specialization. An espin construct bound to F-actin with an approximately threefold higher affinity (K(d) = approximately 70 nM) than small espin and was approximately 2.5 times more efficient at forming bundles. The increased affinity appeared to be due to an additional actin-binding site in the N terminus of espin. This additional actin-binding site bound to F-actin with a K(d) of approximately 1 microM, decorated actin stress fiber-like structures in transfected cells, and was mapped to a peptide between the two proline-rich peptides in the N terminus of espin. Espin was detected at approximately 4-5 x 10(6) copies per ectoplasmic specialization, or approximately 1 espin per 20 actin monomers and accumulated there coincident with the formation of parallel actin bundles during spermiogenesis. These results suggest that espin is a major actin-bundling protein of the Sertoli cell-spermatid ectoplasmic specialization.
Figures
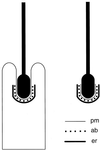
Figure 1
Simplified schematic diagram of an ES formed between a Sertoli cell and a late spermatid in section. pm, Sertoli cell plasma membrane; er, flattened cistern of endoplasmic reticulum; ab, actin bundles. Each dot represents a parallel actin bundle cut in cross section. (Left) In the seminiferous epithelium, the head of the spermatid is held within an invagination of the Sertoli cell, and the ES is found where the Sertoli cell plasma membrane makes close contact with acrosomal region of the spermatid head. (Right) After isolation by mechanical dissociation, the majority of late spermatids retain an ES, including junctional plaque, attached to their head.
Figure 2
Organization of the mouse espin gene. This diagram depicts the relative sizes and positions of the exons deployed differentially to construct small espin and approximately the C-terminal 60% of espin in the mouse. It highlights the positions of the exons that encode the two proline-rich peptides, the potential P-loop, the 66-amino-acid forked homology domain, and the shared C-terminal actin-bundling module (Bartles et al., 1996, 1998). The shaded exons, t and v, are specific to small espin. These sequence data are available from GenBank under accession number AF134858.
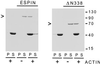
Figure 3
Actin binding and bundling by recombinant espin and ΔN338-espin as revealed by the low-speed centrifugation assay. Shown are Coomassie blue-stained gels of the pellet (P) and supernatant (S) that result from low-speed centrifugation when rabbit skeletal muscle F-actin was incubated alone or in the presence of either recombinant full-length espin for 1 h at 4°C in 0.1 M KCl, 0.1 M imidazole-HCl, 5 mM 2-mercaptoethanol, 1 mM MgCl2, 0.5 mM ATP, 1 mM NaN3, pH 8.5 (left panel), or recombinant ΔN338-espin for 1 h at 37°C in 0.1 M KCl, 10 mM imidazole-HCl, 0.5 mM dithiothreitol, 1 mM MgCl2, 0.5 mM ATP, 1 mM NaN3, pH 7.4 (right panel). The arrowheads at the left denote the position of the recombinant espin construct. The actin is the major band migrating slightly above the 40-kDa marker.
Figure 4
Actin bundling by recombinant espin and ΔN338-espin as revealed by negative staining. Rabbit skeletal muscle actin was incubated alone (A) or in the presence of recombinant full-length espin (C) or recombinant ΔN338-espin (B and D) under the conditions of Figure 3, and aliquots were taken for negative staining. (A–C) Low magnification (bar in D, 770 nm); (D) high magnification (bar, 37 nm).
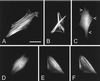
Figure 5
Localization of GFP-espin and GFP-espin(339–720) in transiently transfected BHK cells. (A and B) GFP-espin in living cells. (C and D) GFP-espin(339–720) in living cells. Arrowheads in C denote fine labeled filaments observed in the thinner parts of cell near the periphery. (E and F) GFP-espin(339–720) (E) and rhodamine-phalloidin (F) in fixed and permeabilized cell. Bar in A, 25 μm.
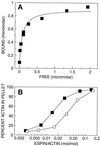
Figure 6
Concentration dependence of actin-binding and -bundling by recombinant ΔN338-espin. (A) Direct plot of bound versus free for the binding of different concentrations of recombinant ΔN338-espin to a fixed concentration of rabbit skeletal muscle F-actin using the high-speed centrifugation actin-binding assay under the conditions of Figure 3, right panel. The curve was obtained by nonlinear least-squares fitting to a rectangular hyperbola. (B) Percent of actin obtained in the pellet in the low-speed-centrifugation actin-bundling assay as increasing concentrations of recombinant ΔN338-espin (solid squares) or recombinant small espin (open circles) were added to a fixed concentration of F-actin.
Figure 7
Actin binding by recombinant espin(339–720), a fragment from the N terminus of espin that is missing the C-terminal actin-bundling module, and the mapping of its actin-binding site by deletion mutagenesis. (A) Coomassie blue-stained gel of the pellet (P) and supernatant (S) that result from high-speed centrifugation when rabbit skeletal muscle F-actin is incubated alone or in the presence of recombinant espin(339–720) for 1 h at 37°C in 0.1 M KCl, 10 mM imidazole-HCl, 0.5 mM dithiothreitol, 1 mM MgCl2, 0.5 mM ATP, 1 mM NaN3, pH 7.4. The arrowhead at the left denotes the position of the recombinant espin construct. The actin is the major band migrating slightly above the 40-kDa marker. (B) Direct plot of bound versus free for the binding of different concentrations of recombinant espin(339–720) to a fixed concentration of rabbit skeletal muscle F-actin under the conditions of A. The curve was obtained by nonlinear least-squares fitting to a rectangular hyperbola. (C) Actin-binding activity of N- or C-terminal deletion constructs of espin(339–720). + or −, presence or absence of saturable actin-binding activity for the designated construct as determined by scanning laser densitometric analysis of the SDS gels that resulted from the high-speed centrifugation actin-binding assay (e.g., see A and B). In the accompanying diagrams, the two proline-rich peptides (Pr) are shaded, and the shared C-terminal actin-bundling module (ABM) present in the ΔN338-espin bar at the top is shown with fine diagonal hatching.
Figure 8
Immunoperoxidase localization of espin during spermiogenesis. Included are portions of cross-sectional profiles of seminiferous tubules containing spermatids in selected steps of spermiogenesis. In each case, the full span of the seminiferous epithelium is shown, from base (lower and/or left side of the panel) to lumen (upper and/or right side of the panel, where letter is shown). Arrowheads point to examples of areas where the brown immunoperoxidase reaction product indicative of espin can be detected at the site of the ES, i.e., near the heads of spermatids, in step 12 (A), step 19 (B), early step 8 (C), or late step 8 (D and E) of spermiogenesis. Spermatids in late step 7 of spermiogenesis, which do not show evidence of significant espin accumulation, occupy the central part of the seminiferous epithelium in B (zone delimited by brackets). Upper bracket in B, 60 μm.
Figure 9
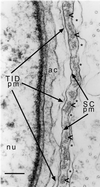
Electron micrograph highlighting the various layers of the ES and neighboring structures present at the site of contact between a Sertoli cell and an early step 8 spermatid in a section of rat testis. The right portion includes the Sertoli cell and highlights its plasma membrane (SC pm) and the parallel actin bundles (arrowheads) and cistern of endoplasmic reticulum (asterisks in lumen) that comprise the ES junctional plaque. Note that the parallel actin bundles are cut in cross section or near cross section, so that each actin filament appears as a small dot. The left portion includes the spermatid and highlights its plasma membrane (TID pm), nucleus (nu), and acrosome (ac). Bar, 0.18 μm.
Similar articles
- Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli.
Bartles JR, Zheng L, Li A, Wierda A, Chen B. Bartles JR, et al. J Cell Biol. 1998 Oct 5;143(1):107-19. doi: 10.1083/jcb.143.1.107. J Cell Biol. 1998. PMID: 9763424 Free PMC article. - Sertoli cell ectoplasmic specializations in the seminiferous epithelium of the testosterone-suppressed adult rat.
O'Donnell L, Stanton PG, Bartles JR, Robertson DM. O'Donnell L, et al. Biol Reprod. 2000 Jul;63(1):99-108. doi: 10.1095/biolreprod63.1.99. Biol Reprod. 2000. PMID: 10859247 - Plastins regulate ectoplasmic specialization via its actin bundling activity on microfilaments in the rat testis.
Li N, Wong CK, Cheng CY. Li N, et al. Asian J Androl. 2016 Sep-Oct;18(5):716-22. doi: 10.4103/1008-682X.166583. Asian J Androl. 2016. PMID: 26608945 Free PMC article. Review. - Tubulobulbar complex: cytoskeletal remodeling to release spermatozoa.
Upadhyay RD, Kumar AV, Ganeshan M, Balasinor NH. Upadhyay RD, et al. Reprod Biol Endocrinol. 2012 Apr 17;10:27. doi: 10.1186/1477-7827-10-27. Reprod Biol Endocrinol. 2012. PMID: 22510523 Free PMC article. Review.
Cited by
- Differential expression of espin isoforms during epithelial morphogenesis, stereociliogenesis and postnatal maturation in the developing inner ear.
Sekerková G, Zheng L, Mugnaini E, Bartles JR. Sekerková G, et al. Dev Biol. 2006 Mar 1;291(1):83-95. doi: 10.1016/j.ydbio.2005.12.021. Epub 2006 Jan 17. Dev Biol. 2006. PMID: 16413524 Free PMC article. - Cytoskeletal dynamics and spermatogenesis.
Lie PP, Mruk DD, Lee WM, Cheng CY. Lie PP, et al. Philos Trans R Soc Lond B Biol Sci. 2010 May 27;365(1546):1581-92. doi: 10.1098/rstb.2009.0261. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20403871 Free PMC article. Review. - Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing.
Kitajiri S, Sakamoto T, Belyantseva IA, Goodyear RJ, Stepanyan R, Fujiwara I, Bird JE, Riazuddin S, Riazuddin S, Ahmed ZM, Hinshaw JE, Sellers J, Bartles JR, Hammer JA 3rd, Richardson GP, Griffith AJ, Frolenkov GI, Friedman TB. Kitajiri S, et al. Cell. 2010 May 28;141(5):786-98. doi: 10.1016/j.cell.2010.03.049. Cell. 2010. PMID: 20510926 Free PMC article. - Whirlin interacts with espin and modulates its actin-regulatory function: an insight into the mechanism of Usher syndrome type II.
Wang L, Zou J, Shen Z, Song E, Yang J. Wang L, et al. Hum Mol Genet. 2012 Feb 1;21(3):692-710. doi: 10.1093/hmg/ddr503. Epub 2011 Nov 2. Hum Mol Genet. 2012. PMID: 22048959 Free PMC article. - p70 S6 kinase and actin dynamics: A perspective.
Ip CK, Wong AS. Ip CK, et al. Spermatogenesis. 2012 Jan 1;2(1):44-52. doi: 10.4161/spmg.19413. Spermatogenesis. 2012. PMID: 22553489 Free PMC article.
References
- Alicea HA, Mooseker MS. Characterization of villin from the intestinal brush border of the rat, and comparative analysis with avian villin. Cell Motil Cytoskeleton. 1988;9:60–72. - PubMed
- Bartles JR, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109:1229–1239. - PubMed
- Bryan J, Kane RE. Separation and interaction of the major components of sea urchin actin gel. J Mol Biol. 1978;125:207–224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases