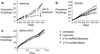Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death - PubMed (original) (raw)
Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death
B Herbert et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The correlation between telomerase activity and human tumors has led to the hypothesis that tumor growth requires reactivation of telomerase and that telomerase inhibitors represent a class of chemotherapeutic agents. Herein, we examine the effects of inhibition of telomerase inside human cells. Peptide nucleic acid and 2'-O-MeRNA oligomers inhibit telomerase, leading to progressive telomere shortening and causing immortal human breast epithelial cells to undergo apoptosis with increasing frequency until no cells remain. Telomere shortening is reversible: if inhibitor addition is terminated, telomeres regain their initial lengths. Our results validate telomerase as a target for the discovery of anticancer drugs and supply general insights into the properties that successful agents will require regardless of chemical type. Chemically similar oligonucleotides are in clinical trials and have well characterized pharmacokinetics, making the inhibitors we describe practical lead compounds for testing for an antitelomerase chemotherapeutic strategy.
Figures
Figure 1
(a) Inhibition of telomerase activity by 2′_-O-_MeRNA oligomers delivered into HME50-5E cells by using FuGENE6 lipid, measured 3 days after transfection. Similar results were observed on introduction of 2′_-O-_MeRNA oligomers into HME50-hTERT cells and on introduction of 2′_-O-_MeRNA or PNA oligomers into DU145 cells. (b) Inhibition of telomerase activity in HME50-5E and DU145 cells by 2′_-O-_MeRNA and PNA oligomers as detected by the TRAP assay. HME50-5E and DU145 cells were collected 1 and 3 days after transfection with 2′_-O-_MeRNA or PNA oligomers. Telomerase activity was quantitated as described (38).
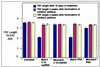
Figure 2
Effects on cell growth after long-term transfection with match or mismatch 2′_-O-_MeRNA. (a) HME50-5E cells transfected with 2′_-O-_MeRNA oligomers. (b) DU145 cells transfected with 2′_-O-_MeRNA oligomers. (c) HME50-hTERT cells transfected with 2′-_O_-MeRNA oligomers.
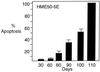
Figure 3
Increase of apoptosis of HME50-5E cells after repeated transfection with fully complementary 2′_-O-_MeRNA. Levels of apoptosis were measured by staining with 4′6-diamidino-2-phenylindole followed by microscopy. Background apoptosis of cells that were untreated or that were treated with 2′_-O-_MeRNA containing mismatched bases was 2–3%. Levels of apoptosis were confirmed by flow cytometry with the ApoAlert Annexin V Apoptosis kit (CLONTECH).
Figure 4
Measurement of telomere restriction fragment length (TRF) in HME50-5E and DU145 cells treated with 2′_-O-_MeRNA and PNA oligomers. (a) HME50-5E cells that had been treated with 2′_-O-_MeRNA oligomers for 60 days, with the results of independent experiments shown in triplicate. (b) DU145 cells treated with 2′_-O-_MeRNA and PNA oligomers for 76 days, with the results of independent experiments shown in triplicate. In parts a and b, the signal intensity in the lanes showing the outcome of treatment with fully complementary oligomer is weak, because telomeres have eroded and few telomeric repeats remain to hybridize with radiolabeled probe. Equivalent amounts of chromosomal DNA were loaded in each lane. TRF lengths are expressed as kilobase pairs.
Figure 5
The TRF length of DU145 as a function of inhibitor addition. TRF lengths were measured after 76 days of addition of fully complementary or mismatch-containing 2′_-O-_MeRNA or PNA oligomers. TRF lengths were then measured again 3 or 5 weeks after terminating oligomer addition. kB, kilobase.
Similar articles
- Telomerase inhibition, telomere shortening, and decreased cell proliferation by cell permeable 2'-O-methoxyethyl oligonucleotides.
Chen Z, Monia BP, Corey DR. Chen Z, et al. J Med Chem. 2002 Dec 5;45(25):5423-5. doi: 10.1021/jm025563v. J Med Chem. 2002. PMID: 12459009 - Effects of oligonucleotide N3'-->P5' thio-phosphoramidate (GRN163) targeting telomerase RNA in human multiple myeloma cells.
Akiyama M, Hideshima T, Shammas MA, Hayashi T, Hamasaki M, Tai YT, Richardson P, Gryaznov S, Munshi NC, Anderson KC. Akiyama M, et al. Cancer Res. 2003 Oct 1;63(19):6187-94. Cancer Res. 2003. PMID: 14559802 Retracted. - Targeting human telomerase in cancer therapy.
Huard S, Autexier C. Huard S, et al. Curr Med Chem Anticancer Agents. 2002 Sep;2(5):577-87. doi: 10.2174/1568011023353822. Curr Med Chem Anticancer Agents. 2002. PMID: 12678725 Review. - Targeting human telomerase by antisense oligonucleotides and ribozymes.
Folini M, Pennati M, Zaffaroni N. Folini M, et al. Curr Med Chem Anticancer Agents. 2002 Sep;2(5):605-12. doi: 10.2174/1568011023353813. Curr Med Chem Anticancer Agents. 2002. PMID: 12678727 Review. - How to inhibit telomerase activity for cancer therapy.
Kyo S, Inoue M. Kyo S, et al. Curr Med Chem Anticancer Agents. 2002 Sep;2(5):613-26. doi: 10.2174/1568011023353796. Curr Med Chem Anticancer Agents. 2002. PMID: 12678728 Review.
Cited by
- Increased Stability of Nucleolar PinX1 in the Presence of TERT.
Keo P, Choi JS, Bae J, Shim YH, Oh BK. Keo P, et al. Mol Cells. 2015 Sep;38(9):814-20. doi: 10.14348/molcells.2015.0144. Epub 2015 Jul 21. Mol Cells. 2015. PMID: 26194824 Free PMC article. - Inhibition of cell proliferation and induction of apoptosis by oleanane triterpenoid (CDDO-Me) in pancreatic cancer cells is associated with the suppression of hTERT gene expression and its telomerase activity.
Deeb D, Gao X, Liu Y, Kim SH, Pindolia KR, Arbab AS, Gautam SC. Deeb D, et al. Biochem Biophys Res Commun. 2012 Jun 15;422(4):561-7. doi: 10.1016/j.bbrc.2012.05.024. Epub 2012 May 16. Biochem Biophys Res Commun. 2012. PMID: 22609405 Free PMC article. - Presence of Endogenous Viral Elements Negatively Correlates with Feline Leukemia Virus Susceptibility in Puma and Domestic Cat Cells.
Chiu ES, VandeWoude S. Chiu ES, et al. J Virol. 2020 Oct 14;94(21):e01274-20. doi: 10.1128/JVI.01274-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32817213 Free PMC article. - The role of telomerase expression and telomere length maintenance in human and mouse.
Weng NP, Hodes RJ. Weng NP, et al. J Clin Immunol. 2000 Jul;20(4):257-67. doi: 10.1023/a:1017223602293. J Clin Immunol. 2000. PMID: 10939713 Review. - Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival.
Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD. Oh H, et al. Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10308-13. doi: 10.1073/pnas.191169098. Epub 2001 Aug 21. Proc Natl Acad Sci U S A. 2001. PMID: 11517337 Free PMC article.
References
- Morin G B. Cell. 1989;59:521–529. - PubMed
- Bryan T M, Cech T R. Curr OpIN Cell Biol. 1999;11:318–324. - PubMed
- Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L C, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. - PubMed
- Autexier C, Greider C W. Trends Biol Sci. 1996;21:387–391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources