Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons - PubMed (original) (raw)
Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons
T Kanamoto et al. Mol Cell Biol. 2000 Jan.
Abstract
We have previously shown that nerve growth factor (NGF) withdrawal-induced death requires the activity of the small GTP-binding protein Cdc42 and that overexpression of an active form of Cdc42 is sufficient to mediate neuronal apoptosis via activation of the c-Jun pathway. Recently, a new mitogen-activated protein (MAP) kinase kinase kinase, apoptosis signal-regulating kinase 1 (ASK1) which activates both the c-Jun N-terminal kinase (JNK) and p38 MAP kinase pathways and plays pivotal roles in tumor necrosis factor- and Fas-induced apoptosis, has been identified. Therefore, we investigated the role of ASK1 in neuronal apoptosis by using rat pheochromocytoma (PC12) neuronal cells and primary rat sympathetic neurons (SCGs). Overexpression of ASK1-DeltaN, a constitutively active mutant of ASK1, activated JNK and induced apoptosis in differentiated PC12 cells and SCG neurons. Moreover, in differentiated PC12 cells, NGF withdrawal induced a four- to fivefold increase in the activity of endogenous ASK1. Finally, expression of a kinase-inactive ASK1 significantly blocked both NGF withdrawal- and Cdc42-induced death and activation of c-jun. Taken together, these results demonstrate that ASK1 is a crucial element of NGF withdrawal-induced activation of the Cdc42-c-Jun pathway and neuronal apoptosis.
Figures
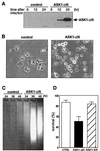
FIG. 1
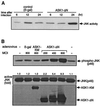
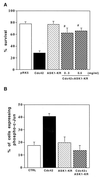
Effect of constitutively active ASK1 on neuronal survival. (A) Expression of ASK1-ΔN in PC12 cells by using recombinant adenoviruses. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-ΔN per cell. At the indicated times after infection, the cells were lysed and immunoblotted with a monoclonal antibody to HA. (B) Induction of neuronal cell death by ASK1-ΔN in NGF-differentiated PC12 cells. The morphology of β-gal-infected cells (left) and ASK1-ΔN-infected cells (right) was examined by phase-contrast microscopy 48 h after infection in the presence of 50 ng of NGF per ml. Original magnification, ×82.5. (C) DNA fragmentation in ASK1-ΔN-infected cells. Soluble cytoplasmic DNA was extracted from 3 × 106 cells after infection with recombinant adenoviruses encoding β-gal or ASK1-ΔN and analyzed by agarose gel electrophoresis. (D) Induction of neuronal cell death by ASK1-ΔN in SCG neurons. SCG neurons, cultured for 5 to 7 days in the presence of NGF, were microinjected with 0.3 mg of ASK1-ΔN (solid box), ASK1-KR (hatched box), or an empty expression vector (empty box) per ml. The 70-kDa Texas Red-dextran was included to mark the injected cells. The number of injected cells was scored 4 to 18 h after injection to allow expression of the proteins of interest (100% value). At 48 h later, the percentage of surviving cells was assessed as described in Materials and Methods. In each experiment, 200 cells were injected. The results are the means of three independent experiments and standard error of the mean.
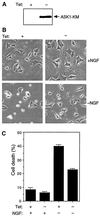
FIG. 2
Effect of ASK1-KM on NGF withdrawal-induced cell death. (A) Expression of ASK1-KM in PC12 cells stably transfected with ASK1-KM (PC12-ASK1-KM cells). PC12-ASK1-KM cells, stably expressing HA-tagged ASK1-KM under the control of a tetracycline-repressible promoter, were cultured in the presence of 50 ng of NGF per ml and 0.5 μg of tetracycline per ml for 9 days. After the additional 24 h of culture in the presence (+) or absence (−) of tetracycline (Tet), the cells were lysed and immunoblotted with anti-HA antibody. (B) Phase-contrast micrographs showing the morphology of PC12-ASK1-KM cells under various culture conditions. PC12-ASK1-KM cells were cultured in the presence of 50 ng of NGF per ml and 0.5 μg of tetracycline per ml for 9 days. After the additional 24 h of culture in the presence (left) or absence (right) of tetracycline (Tet), the medium was changed with (top) or without (bottom) NGF. Cell morphology was examined by phase-contrast microscopy after 48 h. Original magnification, ×82.5. (C) ASK1-KM prevents NGF withdrawal-induced cell death in PC12-ASK1-KM cells. The graph shows the data (mean and standard deviation) for the percentage of apoptotic cells in the experiments in panel B. Apoptotic cells were determined by observing morphological changes with cellular shrinkage or membrane blebbing. The data are averages of five random fields.
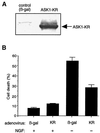
FIG. 3
Effect of ASK1-KR on NGF withdrawal-induced cell death. (A) Expression of ASK1-KR in PC12 cells by using recombinant adenoviruses. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-KR per cell. The cells were lysed and immunoblotted with a monoclonal antibody to HA. (B) Protection of neuronal cell death by ASK1-KR in NGF-differentiated PC12 cells. The morphology of β-gal-infected cells and ASK1-KR-infected cells was examined by phase-contrast microscopy 48 h after infection in the presence of 50 ng of NGF per ml (controls) or in the absence of NGF. The graph shows a quantification of the percent cell death as determined by observing morphological changes with cellular shrinkage or membrane blebbing. The data are averages of five random fields. (C) ASK1-KR prevents NGF withdrawal-induced cell death in SCG neurons. SCG neurons, cultured for 5 to 7 days in the presence of NGF, were microinjected with 70-kDa Texas Red-dextran and increasing concentrations of ASK1-KR (hatched boxes), 0.6 mg of pRK5 (negative control; open box) per ml, or 0.05 mg of Bcl-2 (positive control; solid boxes) per ml. At 24 h later, the cells were deprived of NGF and the number of injected cells was scored (100% value). The percentage of surviving cells was assessed after 48 h as described in Materials and Methods. The results are the means of three independent experiments and standard errors of the mean. (D) ASK1-KR decreases the number of pyknotic nuclei after NGF withdrawal-induced cell death in SCG neurons. SCG neurons were injected with a control empty expression vector or with ASK1-KR and removed from NGF. Uninjected cells, maintained in the presence or the absence of NGF, were included as controls. After 24 h, the nuclear morphology was visualized by Hoechst 33342 staining. The results are the mean of three independent experiments and standard error of the mean. The P value of ASK1-KR at 0.3 mg/ml is <0.01.
FIG. 3
Effect of ASK1-KR on NGF withdrawal-induced cell death. (A) Expression of ASK1-KR in PC12 cells by using recombinant adenoviruses. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-KR per cell. The cells were lysed and immunoblotted with a monoclonal antibody to HA. (B) Protection of neuronal cell death by ASK1-KR in NGF-differentiated PC12 cells. The morphology of β-gal-infected cells and ASK1-KR-infected cells was examined by phase-contrast microscopy 48 h after infection in the presence of 50 ng of NGF per ml (controls) or in the absence of NGF. The graph shows a quantification of the percent cell death as determined by observing morphological changes with cellular shrinkage or membrane blebbing. The data are averages of five random fields. (C) ASK1-KR prevents NGF withdrawal-induced cell death in SCG neurons. SCG neurons, cultured for 5 to 7 days in the presence of NGF, were microinjected with 70-kDa Texas Red-dextran and increasing concentrations of ASK1-KR (hatched boxes), 0.6 mg of pRK5 (negative control; open box) per ml, or 0.05 mg of Bcl-2 (positive control; solid boxes) per ml. At 24 h later, the cells were deprived of NGF and the number of injected cells was scored (100% value). The percentage of surviving cells was assessed after 48 h as described in Materials and Methods. The results are the means of three independent experiments and standard errors of the mean. (D) ASK1-KR decreases the number of pyknotic nuclei after NGF withdrawal-induced cell death in SCG neurons. SCG neurons were injected with a control empty expression vector or with ASK1-KR and removed from NGF. Uninjected cells, maintained in the presence or the absence of NGF, were included as controls. After 24 h, the nuclear morphology was visualized by Hoechst 33342 staining. The results are the mean of three independent experiments and standard error of the mean. The P value of ASK1-KR at 0.3 mg/ml is <0.01.
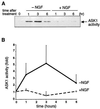
FIG. 4
Activation of endogenous ASK1 by NGF withdrawal. (A) NGF-differentiated PC12 cells were deprived of NGF and further cultured in the absence (−NGF) or presence (+NGF) of NGF for the indicated times. Endogenous ASK1 was immunoprecipitated with anti-ASK1 antibody (DAV). The immune complex was incubated with GST-MKK6 and GST-SAPK3/p38γ, and the kinase activity was measured with ATF2 peptide as a substrate. (B) Relative changes of the kinase activity were calculated by standardizing the background signal as a basal level and plotted to give a graphic representation.
FIG. 5
ASK1-dependent activation of JNK pathway. (A) ASK1-ΔN-dependent activation of endogenous JNK in PC12 cells. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-ΔN per cell. At 24 h after infection, immunoprecipitated endogenous JNK activity was measured with GST–c-Jun as a substrate. The samples were analysed by SDS-PAGE with an image analyzer. (B) Effect of ASK1-ΔN at 45 h. Differentiated PC12 cells were infected with adenoviruses encoding control β-gal, ASK1-KM at 800 PFU/cell, and ASK1-ΔN at the indicated MOIs. At 45 h later, the cells were lysed and subjected to Western blot analysis with specific phospho-JNK (top), JNK (middle), and HA (bottom) antibodies. The fold activation of JNK was standardized to the +NGF control (top panel, left lane). (C) ASK1-dependent phosphorylation of c-Jun in SCG neurons. SCG neurons were microinjected either with 0.3 mg of an empty expression vector or ASK1-ΔN per ml, together with 5 mg of guinea pig IgG per ml to detect the injected cells, and maintained in the presence of NGF or with 0.5 mg of the control empty vector per ml or various concentrations of ASK1-KR and removed from NGF. Controls of uninjected cells were included. After 24 h, the cells were fixed, permeabilized, and stained with a specific anti-phospho–c-Jun antibody. Only the cells in which phospho–c-Jun staining was clearly above background were scored positive. The results are represented as a bar graph and are the mean and standard error of the mean of four independent experiments, (D) ASK1-ΔN-dependent activation of c-jun promoter in SCG neurons. A 0.05-mg/ml concentration of the c-_jun_–CAT reporter gene was microinjected alone or with 0.3 mg of ASK1-ΔN per ml into 5 to 7-day-old SCG neurons, together with 5 mg of guinea pig IgG per ml to detect the injected cells. In the NGF withdrawal experiments, SCG neurons were microinjected with 0.05 mg of the c-_jun_–CAT reporter gene per ml alone or with increasing concentrations of ASK1-KR together with 5 mg of guinea pig IgG per ml to monitor the injected cells. The neurons were either maintained in the presence of NGF or removed from NGF for 24 h. The cells were then fixed, permeabilized, and stained as described in Materials and Methods. Only the cells in which CAT staining was clearly above background were scored positive. The results are presented as a bar graph. The data are the means and standard errors of the mean of three independent experiments. ASK1-ΔN is capable of inducing a twofold increase in the level of CAT expression, whereas ASK1-KR blocks the increase in c-jun activation that is normally observed after NGF withdrawal. (E) FLAGΔ169-Jun blocks ASK1-induced apoptosis. FLAGΔ169-Jun at the indicated concentrations and 0.3 mg of ASK1-ΔN per ml were microinjected into 5- to 7-day old SCG neurons and maintained in the presence of NGF. The percentage of surviving cells was assessed 48 h later. The results are the means and standard errors of the mean of three independent experiments.
FIG. 5
ASK1-dependent activation of JNK pathway. (A) ASK1-ΔN-dependent activation of endogenous JNK in PC12 cells. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-ΔN per cell. At 24 h after infection, immunoprecipitated endogenous JNK activity was measured with GST–c-Jun as a substrate. The samples were analysed by SDS-PAGE with an image analyzer. (B) Effect of ASK1-ΔN at 45 h. Differentiated PC12 cells were infected with adenoviruses encoding control β-gal, ASK1-KM at 800 PFU/cell, and ASK1-ΔN at the indicated MOIs. At 45 h later, the cells were lysed and subjected to Western blot analysis with specific phospho-JNK (top), JNK (middle), and HA (bottom) antibodies. The fold activation of JNK was standardized to the +NGF control (top panel, left lane). (C) ASK1-dependent phosphorylation of c-Jun in SCG neurons. SCG neurons were microinjected either with 0.3 mg of an empty expression vector or ASK1-ΔN per ml, together with 5 mg of guinea pig IgG per ml to detect the injected cells, and maintained in the presence of NGF or with 0.5 mg of the control empty vector per ml or various concentrations of ASK1-KR and removed from NGF. Controls of uninjected cells were included. After 24 h, the cells were fixed, permeabilized, and stained with a specific anti-phospho–c-Jun antibody. Only the cells in which phospho–c-Jun staining was clearly above background were scored positive. The results are represented as a bar graph and are the mean and standard error of the mean of four independent experiments, (D) ASK1-ΔN-dependent activation of c-jun promoter in SCG neurons. A 0.05-mg/ml concentration of the c-_jun_–CAT reporter gene was microinjected alone or with 0.3 mg of ASK1-ΔN per ml into 5 to 7-day-old SCG neurons, together with 5 mg of guinea pig IgG per ml to detect the injected cells. In the NGF withdrawal experiments, SCG neurons were microinjected with 0.05 mg of the c-_jun_–CAT reporter gene per ml alone or with increasing concentrations of ASK1-KR together with 5 mg of guinea pig IgG per ml to monitor the injected cells. The neurons were either maintained in the presence of NGF or removed from NGF for 24 h. The cells were then fixed, permeabilized, and stained as described in Materials and Methods. Only the cells in which CAT staining was clearly above background were scored positive. The results are presented as a bar graph. The data are the means and standard errors of the mean of three independent experiments. ASK1-ΔN is capable of inducing a twofold increase in the level of CAT expression, whereas ASK1-KR blocks the increase in c-jun activation that is normally observed after NGF withdrawal. (E) FLAGΔ169-Jun blocks ASK1-induced apoptosis. FLAGΔ169-Jun at the indicated concentrations and 0.3 mg of ASK1-ΔN per ml were microinjected into 5- to 7-day old SCG neurons and maintained in the presence of NGF. The percentage of surviving cells was assessed 48 h later. The results are the means and standard errors of the mean of three independent experiments.
FIG. 5
ASK1-dependent activation of JNK pathway. (A) ASK1-ΔN-dependent activation of endogenous JNK in PC12 cells. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-ΔN per cell. At 24 h after infection, immunoprecipitated endogenous JNK activity was measured with GST–c-Jun as a substrate. The samples were analysed by SDS-PAGE with an image analyzer. (B) Effect of ASK1-ΔN at 45 h. Differentiated PC12 cells were infected with adenoviruses encoding control β-gal, ASK1-KM at 800 PFU/cell, and ASK1-ΔN at the indicated MOIs. At 45 h later, the cells were lysed and subjected to Western blot analysis with specific phospho-JNK (top), JNK (middle), and HA (bottom) antibodies. The fold activation of JNK was standardized to the +NGF control (top panel, left lane). (C) ASK1-dependent phosphorylation of c-Jun in SCG neurons. SCG neurons were microinjected either with 0.3 mg of an empty expression vector or ASK1-ΔN per ml, together with 5 mg of guinea pig IgG per ml to detect the injected cells, and maintained in the presence of NGF or with 0.5 mg of the control empty vector per ml or various concentrations of ASK1-KR and removed from NGF. Controls of uninjected cells were included. After 24 h, the cells were fixed, permeabilized, and stained with a specific anti-phospho–c-Jun antibody. Only the cells in which phospho–c-Jun staining was clearly above background were scored positive. The results are represented as a bar graph and are the mean and standard error of the mean of four independent experiments, (D) ASK1-ΔN-dependent activation of c-jun promoter in SCG neurons. A 0.05-mg/ml concentration of the c-_jun_–CAT reporter gene was microinjected alone or with 0.3 mg of ASK1-ΔN per ml into 5 to 7-day-old SCG neurons, together with 5 mg of guinea pig IgG per ml to detect the injected cells. In the NGF withdrawal experiments, SCG neurons were microinjected with 0.05 mg of the c-_jun_–CAT reporter gene per ml alone or with increasing concentrations of ASK1-KR together with 5 mg of guinea pig IgG per ml to monitor the injected cells. The neurons were either maintained in the presence of NGF or removed from NGF for 24 h. The cells were then fixed, permeabilized, and stained as described in Materials and Methods. Only the cells in which CAT staining was clearly above background were scored positive. The results are presented as a bar graph. The data are the means and standard errors of the mean of three independent experiments. ASK1-ΔN is capable of inducing a twofold increase in the level of CAT expression, whereas ASK1-KR blocks the increase in c-jun activation that is normally observed after NGF withdrawal. (E) FLAGΔ169-Jun blocks ASK1-induced apoptosis. FLAGΔ169-Jun at the indicated concentrations and 0.3 mg of ASK1-ΔN per ml were microinjected into 5- to 7-day old SCG neurons and maintained in the presence of NGF. The percentage of surviving cells was assessed 48 h later. The results are the means and standard errors of the mean of three independent experiments.
FIG. 6
Effect of a dominant negative ASK1 on Cdc42-induced cell death. (A) Cdc42-induced apoptosis requires ASK1 activity. We coinjected 0.3 or 0.5 mg of ASK1-KR per ml, 0.1 mg of V12Cdc42 per ml, and 70-kDa Texas Red-dextran into SCG neurons. The cells were maintained in the presence of NGF, and the percentage of surviving cells was assessed 48 h after injection as previously described. The results are the means and standard errors of the mean of three independent experiments. ASK1-KR blocks Cdc42-induced death. The P value of ASK1-KR is <0.01 at 0.3 mg/ml and <0.001 at 0.5 mg/ml. (B) ASK1-KR blocks Cdc42-induced increase of phosphorylation of c-Jun. SCG neurons were coinjected with 0.5 mg of ASK1-KR per ml and 0.1 mg of V12Cdc42 per ml together with 5 mg of guinea pig IgG per ml to detect the injected cells and maintained in the presence of NGF. The cells were stained 24 h later with a specific anti-phospho–c-Jun antibody. The results are the means and standard errors of the mean of three independent experiments. The P value of ASK1-KR at 0.5 mg/ml is <0.01.
FIG. 7
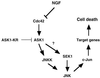
Model for the apoptotic signalling pathway mediated by NGF withdrawal. Removal of the survival agent, NGF, activates the small GTPase Cdc42. Activated Cdc42 leads to activation of ASK1 which induces an increase in JNK activity. Presumably, phosphorylation of c-Jun activates its transcriptional activity, which then turn on the transcription of genes necessary for neuronal apoptosis to proceed.
Similar articles
- Evidence for a role of mixed lineage kinases in neuronal apoptosis.
Mota M, Reeder M, Chernoff J, Bazenet CE. Mota M, et al. J Neurosci. 2001 Jul 15;21(14):4949-57. doi: 10.1523/JNEUROSCI.21-14-04949.2001. J Neurosci. 2001. PMID: 11438570 Free PMC article. - The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis.
Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. Xu Z, et al. Mol Cell Biol. 2001 Jul;21(14):4713-24. doi: 10.1128/MCB.21.14.4713-4724.2001. Mol Cell Biol. 2001. PMID: 11416147 Free PMC article. - Apoptosis-signal regulating kinase-1 is involved in the low potassium-induced activation of p38 mitogen-activated protein kinase and c-Jun in cultured cerebellar granule neurons.
Yamagishi S, Yamada M, Koshimizu H, Takai S, Hatanaka H, Takeda K, Ichijo H, Shimoke K, Ikeuchi T. Yamagishi S, et al. J Biochem. 2003 Jun;133(6):719-24. doi: 10.1093/jb/mvg092. J Biochem. 2003. PMID: 12869527 - Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice.
Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Matsuzawa A, et al. Antioxid Redox Signal. 2002 Jun;4(3):415-25. doi: 10.1089/15230860260196218. Antioxid Redox Signal. 2002. PMID: 12215209 Review. - Roles of MAPKKK ASK1 in stress-induced cell death.
Takeda K, Matsuzawa A, Nishitoh H, Ichijo H. Takeda K, et al. Cell Struct Funct. 2003 Feb;28(1):23-9. doi: 10.1247/csf.28.23. Cell Struct Funct. 2003. PMID: 12655147 Review.
Cited by
- Therapeutic targets in the ASK1-dependent stress signaling pathways.
Hayakawa R, Hayakawa T, Takeda K, Ichijo H. Hayakawa R, et al. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(8):434-53. doi: 10.2183/pjab.88.434. Proc Jpn Acad Ser B Phys Biol Sci. 2012. PMID: 23060232 Free PMC article. Review. - Arsenite-induced apoptosis in cortical neurons is mediated by c-Jun N-terminal protein kinase 3 and p38 mitogen-activated protein kinase.
Namgung U, Xia Z. Namgung U, et al. J Neurosci. 2000 Sep 1;20(17):6442-51. doi: 10.1523/JNEUROSCI.20-17-06442.2000. J Neurosci. 2000. PMID: 10964950 Free PMC article. - Apoptosis signal-regulating kinase 1 mediates denbinobin-induced apoptosis in human lung adenocarcinoma cells.
Kuo CT, Chen BC, Yu CC, Weng CM, Hsu MJ, Chen CC, Chen MC, Teng CM, Pan SL, Bien MY, Shih CH, Lin CH. Kuo CT, et al. J Biomed Sci. 2009 May 1;16(1):43. doi: 10.1186/1423-0127-16-43. J Biomed Sci. 2009. PMID: 19405983 Free PMC article. - Targeted delivery of brain-derived neurotrophic factor for the treatment of blindness and deafness.
Khalin I, Alyautdin R, Kocherga G, Bakar MA. Khalin I, et al. Int J Nanomedicine. 2015 Apr 30;10:3245-67. doi: 10.2147/IJN.S77480. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25995632 Free PMC article. Review. - Impact of opiate-HIV-1 interactions on neurotoxic signaling.
Hauser KF, El-Hage N, Buch S, Nath A, Tyor WR, Bruce-Keller AJ, Knapp PE. Hauser KF, et al. J Neuroimmune Pharmacol. 2006 Mar;1(1):98-105. doi: 10.1007/s11481-005-9000-4. J Neuroimmune Pharmacol. 2006. PMID: 18040795 Review.
References
- Berestetskaya Y V, Faure M P, Ichijo H, Voyno-Yasenetskaya T A. Regulation of apoptosis by alpha-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1. J Biol Chem. 1998;273:27816–27823. - PubMed
- Brown J L, Stowers L B, Trejo J A, Coughlin S, Chant J. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr Biol. 1996;6:598–605. - PubMed
- Chang H Y, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. - PubMed
- Clarke P G. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous