Induction of cell scattering by expression of beta1 integrins in beta1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function - PubMed (original) (raw)
Induction of cell scattering by expression of beta1 integrins in beta1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function
C Gimond et al. J Cell Biol. 1999.
Abstract
Adhesion receptors, which connect cells to each other and to the surrounding extracellular matrix (ECM), play a crucial role in the control of tissue structure and of morphogenesis. In this work, we have studied how intercellular adhesion molecules and beta1 integrins influence each other using two different beta1-null cell lines, epithelial GE11 and fibroblast-like GD25 cells. Expression of beta1A or the cytoplasmic splice variant beta1D, induced the disruption of intercellular adherens junctions and cell scattering in both GE11 and GD25 cells. In GE11 cells, the morphological change correlated with the redistribution of zonula occluden (ZO)-1 from tight junctions to adherens junctions at high cell confluency. In addition, the expression of beta1 integrins caused a dramatic reorganization of the actin cytoskeleton and of focal contacts. Interaction of beta1 integrins with their respective ligands was required for a complete morphological transition towards the spindle-shaped fibroblast-like phenotype. The expression of an interleukin-2 receptor (IL2R)-beta1A chimera and its incorporation into focal adhesions also induced the disruption of cadherin-based adhesions and the reorganization of ECM-cell contacts, but failed to promote cell migration on fibronectin, in contrast to full-length beta1A. This indicates that the disruption of cell-cell adhesion is not simply the consequence of the stimulated cell migration. Expression of beta1 integrins in GE11 cells resulted in a decrease in cadherin and alpha-catenin protein levels accompanied by their redistribution from the cytoskeleton-associated fraction to the detergent-soluble fraction. Regulation of alpha-catenin protein levels by beta1 integrins is likely to play a role in the morphological transition, since overexpression of alpha-catenin in GE11 cells before beta1 prevented the disruption of intercellular adhesions and cell scattering. In addition, using biochemical activity assays for Rho-like GTPases, we show that the expression of beta1A, beta1D, or IL2R-beta1A in GE11 or GD25 cells triggers activation of both RhoA and Rac1, but not of Cdc42. Moreover, dominant negative Rac1 (N17Rac1) inhibited the disruption of cell-cell adhesions when expressed before beta1. However, all three GTPases might be involved in the morphological transition, since expression of either N19RhoA, N17Rac1, or N17Cdc42 reversed cell scattering and partially restored cadherin-based adhesions in GE11-beta1A cells. Our results indicate that beta1 integrins regulate the polarity and motility of epithelial cells by the induction of intracellular molecular events involving a downregulation of alpha-catenin function and the activation of the Rho-like G proteins Rac1 and RhoA.
Figures
Figure 1
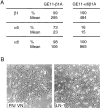
Morphological changes induced by the expression of β1A or β1D in GE11 and GD25 cells. GE11 and GD25 cells were transduced with either the empty LZRS vector (GE11-control and GD25-control), the LZRS vector coding for the full-length β1A (GE11-β1A and GD25-β1A), or full-length β1D integrin splice variant (GE11-β1D and GD25-β1D). Photographs of cells stably expressing β1A or β1D were taken by phase-contrast microscopy after zeocin selection.
Figure 2
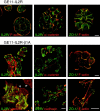
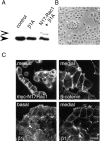
Distribution of F-actin and various adhesion structures in GE11-control and GE11-β1A cells. Cells were grown for 2 d on glass coverslips, and after fixation and Triton X-100 permeabilization, stained as indicated for F-actin with rhodamine-labeled phalloidin or double-stained for β1A and F-actin, vinculin and cadherin, vinculin and F-actin, and ZO-1 and F-actin. Cells were visualized by confocal laser-scanning microscopy. Basal and medial focus planes are as indicated. Bar, 20 μm.
Figure 3
Scattering of GE11 cells is dependent on β1 integrin-ligand interaction. (A) Expression of β1, α5, and α6 integrin subunits in GE11-β1 and GE11-α6β1 cells overexpressing the α6β1 integrin, as determined by FACS® analysis. Both the percentage of cells expressing the different subunits and their expression levels in positive cells in mean fluorescence (arbitrary units) are indicated. (B) Phase-contrast microscopy of GE11-α6β1 grown on plastic in the presence of FCS (FN/VN) or on laminin-1 substratum (LN-1).
Figure 4
Expression of IL2R-β1A induces the disruption of cadherin-based intercellular adhesions, and increases the size of focal contacts in GE11 cells. Stably transduced GE11-IL2R and GE11-IL2R-β1A cells were grown for 2 d on glass coverslips, and after fixation and Triton X-100 permeabilization, analyzed by double immunofluoresence for the expression of either IL2R together with F-actin (revealed by staining with rhodamine-phalloidin), α-catenin, vinculin, or cadherin, or for the expression of ZO-1 together with F-actin. Arrows indicate IL2R-β1A–negative cells that exhibit a vinculin-positive ring of focal contacts. Bars, 20 μm.
Figure 5
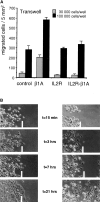
(A) Expression of β1A, but not that of IL2R-β1A, enhances GE11 cell migration through fibronectin-coated Transwell filters. Fibronectin was coated on the lower side of the filter, and 3 × 104 or 105 cells were seeded in the upper compartment of the Transwell, after which cells were allowed to migrate for 2 h. Cells that remained on the upper side of the filter were removed by washing, and cells that had migrated to the lower side of the filter were fixed and stained with crystal violet. Cells were counted on photographs taken from three different fields (5 mm2) and the results were averaged. Error bars represent SEMs. (B) Scratch assay of GE11-control and GE11-β1A cells. Cells were seeded at high density on plastic under standard culture conditions for 2 h. Subsequently, a cross was scratched to facilitate the marking of the cells. Phase-contrast micrographs were taken at the indicated timepoints. The white bars represent the progression of migrating cells.
Figure 6
Expression of cadherin, α-, and β-catenin and their association with the cytoskeleton is reduced in GE11-β1A and GE11-β1D cells as compared with that in GE11-control cells. Cadherin and catenin Triton X-100 solubility was assayed on immunoblots containing proteins (50 μg protein) from total cell lysates and from 1% Triton X-100–soluble (S) and -insoluble (I) fractions of GE11-control, GE11-β1A, and GE11-β1D cells. Immunoblots were probed for pan-cadherin, α-catenin, and β-catenin.
Figure 7
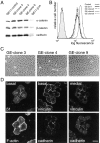
Overexpression of α-catenin prevents the morphological change induced by β1 integrins. (A) Immunoblot analysis showing the levels of α-, β-catenin, and cadherins in cell lysates (50 μg protein) prepared from GE11 control cells, three GE11–α-catenin cell clones stably expressing β1A (GE clones 3, 4, and 9), and GE11-β1A cells. (B) Cell surface expression of β1 on the same cells as above. Flow cytometry analysis of cells was carried out with the TS2/16 anti-β1 mAb, followed by incubation with FITC-conjugated anti–mouse IgG. (C) Phase-contrast microscopy of GE11–α-catenin–β1A cell clones. Clones 3 and 9 exhibit an epithelial phenotype, whereas clone 4 appeared more fibloblast-like. (D) Double-immunofluorescence staining of GE11–clone 9 cells. Cells were fixed and immunostained for β1A and F-actin and for vinculin and cadherin. Basal and medial focal planes are as indicated. Bar, 20 μm.
Figure 8
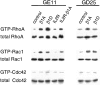
Expression of β1 integrins in GE11 and GD25 cells activates RhoA and Rac1 but not Cdc42. Lysates of GE11 and GD25 cells expressing either the control vector alone, β1A, β1D, IL2R, or IL2R-β1A were incubated with GST-rhotekin fusion protein for the RhoA assay, or with GST-PAK fusion protein for the Rac1 and Cdc42 assays. The presence of active, GTP-bound RhoA, Rac1, or Cdc42 was analyzed by immunoblotting. Total amounts of RhoA, Rac1, and Cdc42 were determined in total cell lysates.
Figure 9
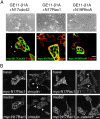
Dominant negative Rac1 (N17Rac1) prevents the disruption of intercellular adhesions and cell scattering induced by β1 integrins. (A) Total amounts of endogenous Rac1 in GE11-control, GE11-β1A, and of Rac1 and N17Rac1 in GE11-N17Rac1-β1A cells were determined by immunoblotting. Blots were probed with anti-Rac1 mAb. The open arrow indicates myc-N17Rac1 and the closed arrow indicates endogenous Rac1. (B) Phase-contrast microscopy of GE11-N17Rac1-β1A cells. (C) Immunofluorescence staining of GE11-N17Rac1-β1A cells for expression of myc epitope-tagged N17Rac1, β-catenin, and β1 integrins. Basal and medial focal planes are as indicated. Bar, 20 μm.
Figure 10
Expression of dominant negative mutants of Cdc42 (N17Cdc42), Rac1 (N17Rac1), or RhoA (N19RhoA) in GE11-β1A cells inhibits cell scattering and partially restores cadherin-based cell–cell adhesions. (A) Phase-contrast and fluorescence micrographs of GE11-β1A cells expressing either N17Cdc42, N17Rac1, or N19RhoA. Cells were photographed 3 d after infection. Double-immunofluorescence staining of cells was for myc-tagged N17Cdc42, N17Rac1, and N19RhoA using anti-myc mAb and rhodamine-phalloidin for detection of F-actin distribution. (B) Double-immunofluorescence staining of GE11-β1A-N17Rac1 cells. GE11-β1A cells were grown on coverslips and N17Rac1 was introduced by retroviral transduction. 3 d after infection, cells were fixed and analyzed by double-immunfluoresence for expression of myc-tagged N17Rac1 together with either vinculin, β1A, or α-catenin. Basal and medial focal planes are as indicated. Arrows indicate the localization of vinculin or β1 in N17Rac1-positive cells. Bar, 20 μm.
Figure 11
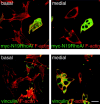
Loss of actin stress fibers and focal contacts in GE11-β1A cells expressing N19-RhoA. Double-immunofluorescence staining of GE11-β1A cells, 3–4 d after N19RhoA retroviral transduction. Cells were fixed and double-stained for either myc-tagged N19RhoA (upper panels) or vinculin (lower panels) with F-actin. Note that the cluster of four cells in the upper panels that express N19RhoA at high levels does not exhibit actin stress fibers, in contrast to the cells expressing no or little N19RhoA. The cluster of seven cells shown in the lower panels displays no vinculin-positive focal contacts. Basal and medial focal planes are as indicated. Bar, 20 μm.
Similar articles
- Temporal and spatial modulation of Rho GTPases during in vitro formation of capillary vascular network. Adherens junctions and myosin light chain as targets of Rac1 and RhoA.
Cascone I, Giraudo E, Caccavari F, Napione L, Bertotti E, Collard JG, Serini G, Bussolino F. Cascone I, et al. J Biol Chem. 2003 Dec 12;278(50):50702-13. doi: 10.1074/jbc.M307234200. Epub 2003 Sep 12. J Biol Chem. 2003. PMID: 12972426 - RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin.
Bruewer M, Hopkins AM, Hobert ME, Nusrat A, Madara JL. Bruewer M, et al. Am J Physiol Cell Physiol. 2004 Aug;287(2):C327-35. doi: 10.1152/ajpcell.00087.2004. Epub 2004 Mar 24. Am J Physiol Cell Physiol. 2004. PMID: 15044152 - p120 catenin regulates the actin cytoskeleton via Rho family GTPases.
Noren NK, Liu BP, Burridge K, Kreft B. Noren NK, et al. J Cell Biol. 2000 Aug 7;150(3):567-80. doi: 10.1083/jcb.150.3.567. J Cell Biol. 2000. PMID: 10931868 Free PMC article. - Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion.
Arthur WT, Noren NK, Burridge K. Arthur WT, et al. Biol Res. 2002;35(2):239-46. doi: 10.4067/s0716-97602002000200016. Biol Res. 2002. PMID: 12415742 Review. - Rho-family GTPases in cadherin-mediated cell-cell adhesion.
Fukata M, Kaibuchi K. Fukata M, et al. Nat Rev Mol Cell Biol. 2001 Dec;2(12):887-97. doi: 10.1038/35103068. Nat Rev Mol Cell Biol. 2001. PMID: 11733768 Review.
Cited by
- Down-regulation of alpha v/beta 3 integrin via misrouting to lysosomes by overexpression of a beta 3Lamp1 fusion protein.
Conesa M, Prat A, Mort JS, Marvaldi J, Lissitzky JC, Seidah NG. Conesa M, et al. Biochem J. 2003 Mar 1;370(Pt 2):703-11. doi: 10.1042/BJ20021301. Biochem J. 2003. PMID: 12444923 Free PMC article. - Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner.
Jiménez-Soto LF, Kutter S, Sewald X, Ertl C, Weiss E, Kapp U, Rohde M, Pirch T, Jung K, Retta SF, Terradot L, Fischer W, Haas R. Jiménez-Soto LF, et al. PLoS Pathog. 2009 Dec;5(12):e1000684. doi: 10.1371/journal.ppat.1000684. Epub 2009 Dec 4. PLoS Pathog. 2009. PMID: 19997503 Free PMC article. - "Pinopodes" and implantation.
Lopata A, Bentin-Ley U, Enders A. Lopata A, et al. Rev Endocr Metab Disord. 2002 May;3(2):77-86. doi: 10.1023/a:1015455709833. Rev Endocr Metab Disord. 2002. PMID: 12007284 Review. No abstract available. - Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell-cell adhesion in normal human keratinocytes.
Braga VM, Betson M, Li X, Lamarche-Vane N. Braga VM, et al. Mol Biol Cell. 2000 Nov;11(11):3703-21. doi: 10.1091/mbc.11.11.3703. Mol Biol Cell. 2000. PMID: 11071901 Free PMC article.
References
- Akiyama S.K., Yamada S.S., Yamada K.M., LaFlamme S.E. Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J. Biol.Chem. 1994;269:15961–15964. - PubMed
- Barry S.T., Flinn H.M., Humphries M.J., Critchley D.R., Ridley A.J. Requirement for rho in integrin signaling. Cell Adhes. Commun. 1997;4:387–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous