Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes - PubMed (original) (raw)
Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes
V Lukyanenko et al. J Physiol. 1999.
Abstract
1. We carried out confocal Ca2+ imaging in myocytes permeabilized with saponin in 'internal' solutions containing: MgATP, EGTA and fluo-3 potassium salt. 2. Permeabilized myocytes exhibited spontaneous Ca2+ sparks and waves similar to those observed in intact myocytes loaded with fluo-3 AM. 3. In the presence of 'low' [EGTA] (0.05 mM), Ca2+ waves arose regularly, even at relatively low [Ca2+] (50-100 nM, free). Increasing [EGTA] resulted in decreased frequency and propagation velocity of Ca2+ waves. Propagating waves were completely abolished at [EGTA] > 0.3 mM. 4. The frequency of sparks increased as a function of [Ca2+] (50-400 nM range) with no sign of a high affinity Ca2+-dependent inactivation process. 5. The rate of occurrence of Ca2+ sparks was increased by calmodulin and cyclic adenosine diphosphate-ribose (cADPR).
Figures
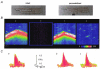
Figure 1. The effects of permeabilization on Ca2+ sparks and Ca2+ waves in ventricular myocytes
A, images of a cardiac myocyte obtained in transmitted light before and after permeabilization with saponin. B, line-scan images of fluorescence in a portion of the same cell pre-loaded with fluo-3 AM measured before permeabilization (a, [Ca2+]o= 5 m
m
), after permeabilization in an internal solution with no dye (b) and after addition to the internal solution 30 μ
m
fluo-3 potassium salt in the presence of 0.1 (c) or 0.5 m
m
EGTA (d) (pCa 7). Calibration bars: horizontal 10 μm, vertical 0.4 s, the colour bar represents changes in units of absolute fluorescence. C, surface plots of Ca2+ sparks measured before permeabilization (a) and after permeabilization in the presence of 0.1 or 0.5 m
m
EGTA (b and c, respectively). Each plot was obtained by averaging 10 individual events.
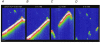
Figure 2. Effects of calcium buffering on Ca2+ waves in saponin-permeabilized myocytes
A, B, C and D, representative line-scan images of fluorescence recorded in a permeabilized myocyte in the presence of 0.05 m
m
(A), 0.1 m
m
(B), 0.2 m
m
(C) and 0.3 m
m
EGTA (D). Free [Ca2+] in all cases was adjusted to 100 n
m
. Calibration bars: horizontal 15 μm, vertical 0.35 s.
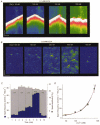
Figure 3. Effects of [Ca2+]i on Ca2+ sparks and Ca2+ waves in saponin-permeabilized myocytes
A, representative line-scan images of fluorescence recorded in a permeabilized myocyte at various [Ca2+]i levels (indicated at the top of the respective images) in the presence of 0.1 m
m
EGTA. B, line-scan images of Ca2+ sparks corrected for increases of background fluorescence at various [Ca2+]i levels (indicated at the top of the respective images) in the presence of 0.5 m
m
EGTA. Calibration bars: horizontal 15 μm (A) and 20 μm (B), vertical 0.5 s (A) and 0.1 s (B). C, Ca2+ spark frequency (blue) and amplitude (light grey) as a function of time before and after elevating [Ca2+]i to indicated levels for the experiment shown in B. D, Ca2+ spark frequency as a function of [Ca2+]i. The values are represented as means ±
s.e.m.
obtained in 5 experiments. The lines were obtained by fitting the data according to the equation _f_=_f_max{[Ca2+]n/([Ca2+]n+K_D_n)}, where _f_max= 10 000 events s−1 (100 μm)−1, _K_D= 9.9 μ
m
and _n_= 1.6 (blue line); _f_max= 20 000 events s−1 (100 μm)−1; _K_D= 15 μ
m
and _n_= 1.6 (red line); and _f_max= 30 000 events s−1 (100 μm)−1; _K_D= 20 μ
m
and _n_= 1.6 (green line).
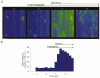
Figure 4. The effects of elevating [Ca2+]i on sparking activity in the presence of thapsigargin
A, representative line-scan images of fluorescence acquired before (a and b) and at different times (2 and 3 min) after increasing [Ca2+]i from 80 n
m
to 250 n
m
in the presence of thapsigargin (c and d, respectively). Thapsigargin (10 μ
m
) was introduced into the bathing solution 1 min before elevating [Ca2+]i. Calibration bars: horizontal 20 μm, vertical 0.15 s. B, Ca2+ spark frequency as a function of time before and after the addition of thapsigargin into the bathing solution in the same experiment. The experimental protocol is presented schematically at the top.
Figure 5. Effects of calmodulin and cADPR on Ca2+ sparks
A, representative line-scan images of fluorescence changes acquired under control conditions (left-hand panel), 15 min after exposure of the cells to 5 μ
m
calmodulin (middle panel), and 10 min after changing back to the control solution (right-hand panel). Calibration bars: horizontal 10 μm, vertical 0.2 s. B, caffeine-induced Ca2+ transients measured in the same cell at the same stages of the experiment as in A. Caffeine (20 m
m
) was applied for 2 s. C, representative line-scan images of fluorescence changes measured under control conditions (left-hand panel), 2 min after exposure of the cells to 5 μ
m
cADPR (middle panel), and 5 min after reverting back to the control solution (right-hand panel). Calibration bars: horizontal 10 μm, vertical 0.2 s.
Similar articles
- Effect of osmotic stress on spontaneous calcium sparks in rat ventricular myocytes.
Xie H, Zhu PH. Xie H, et al. Acta Pharmacol Sin. 2006 Jul;27(7):877-87. doi: 10.1111/j.1745-7254.2006.00371.x. Acta Pharmacol Sin. 2006. PMID: 16787572 - Potentiation of Ca(2+) release by cADP-ribose in the heart is mediated by enhanced SR Ca(2+) uptake into the sarcoplasmic reticulum.
Lukyanenko V, Györke I, Wiesner TF, Györke S. Lukyanenko V, et al. Circ Res. 2001 Sep 28;89(7):614-22. doi: 10.1161/hh1901.098066. Circ Res. 2001. PMID: 11577027 - Effects of photoreleased cADP-ribose on calcium transients and calcium sparks in myocytes isolated from guinea-pig and rat ventricle.
Cui Y, Galione A, Terrar DA. Cui Y, et al. Biochem J. 1999 Sep 1;342 ( Pt 2)(Pt 2):269-73. Biochem J. 1999. PMID: 10455010 Free PMC article. - Sodium-calcium exchange is essential for effective triggering of calcium release in mouse heart.
Neco P, Rose B, Huynh N, Zhang R, Bridge JH, Philipson KD, Goldhaber JI. Neco P, et al. Biophys J. 2010 Aug 4;99(3):755-64. doi: 10.1016/j.bpj.2010.04.071. Biophys J. 2010. PMID: 20682252 Free PMC article. - Calmodulin and cyclic ADP-ribose interaction in Ca2+ signaling related to cardiac sarcoplasmic reticulum: superoxide anion radical-triggered Ca2+ release.
Okabe E, Tsujimoto Y, Kobayashi Y. Okabe E, et al. Antioxid Redox Signal. 2000 Spring;2(1):47-54. doi: 10.1089/ars.2000.2.1-47. Antioxid Redox Signal. 2000. PMID: 11232599 Review.
Cited by
- Modeling Calcium Cycling in the Heart: Progress, Pitfalls, and Challenges.
Qu Z, Yan D, Song Z. Qu Z, et al. Biomolecules. 2022 Nov 14;12(11):1686. doi: 10.3390/biom12111686. Biomolecules. 2022. PMID: 36421700 Free PMC article. Review. - Construction of calcium release sites in cardiac myocytes.
Zahradníková A, Zahradník I. Zahradníková A, et al. Front Physiol. 2012 Aug 20;3:322. doi: 10.3389/fphys.2012.00322. eCollection 2012. Front Physiol. 2012. PMID: 22934071 Free PMC article. - Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass.
Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollmann BC. Hilliard FA, et al. J Mol Cell Cardiol. 2010 Feb;48(2):293-301. doi: 10.1016/j.yjmcc.2009.10.005. Epub 2009 Oct 14. J Mol Cell Cardiol. 2010. PMID: 19835880 Free PMC article. - Probing the outer mitochondrial membrane in cardiac mitochondria with nanoparticles.
Salnikov V, Lukyánenko YO, Frederick CA, Lederer WJ, Lukyánenko V. Salnikov V, et al. Biophys J. 2007 Feb 1;92(3):1058-71. doi: 10.1529/biophysj.106.094318. Epub 2006 Nov 10. Biophys J. 2007. PMID: 17098804 Free PMC article. - Magnesium Ions Moderate Calcium-Induced Calcium Release in Cardiac Calcium Release Sites by Binding to Ryanodine Receptor Activation and Inhibition Sites.
Iaparov B, Baglaeva I, Zahradník I, Zahradníková A. Iaparov B, et al. Front Physiol. 2022 Jan 25;12:805956. doi: 10.3389/fphys.2021.805956. eCollection 2021. Front Physiol. 2022. PMID: 35145426 Free PMC article.
References
- Bassani JW, Bassani RA, Bers DM. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. American Journal of Physiology. 1993;265:C533–540. - PubMed
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca2+ release is regulated by trigger Ca2+ and SR Ca2+ content in cardiac myocytes. American Journal of Physiology. 1995;268:C1313–1329. - PubMed
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991.
- Chamberlain BK, Volpe P, Fleischer S. Calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. Journal of Biological Chemistry. 1984;259:7540–7546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous