HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression - PubMed (original) (raw)
Clinical Trial
. 1999 Dec 21;96(26):15109-14.
doi: 10.1073/pnas.96.26.15109.
N Bhat, C Yoder, T W Chun, J A Metcalf, R Dewar, V Natarajan, R A Lempicki, J W Adelsberger, K D Miller, J A Kovacs, M A Polis, R E Walker, J Falloon, H Masur, D Gee, M Baseler, D S Dimitrov, A S Fauci, H C Lane
Affiliations
- PMID: 10611346
- PMCID: PMC24781
- DOI: 10.1073/pnas.96.26.15109
Clinical Trial
HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression
R T Davey Jr et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Identifying the immunologic and virologic consequences of discontinuing antiretroviral therapy in HIV-infected patients is of major importance in developing long-term treatment strategies for patients with HIV-1 infection. We designed a trial to characterize these parameters after interruption of highly active antiretroviral therapy (HAART) in patients who had maintained prolonged viral suppression on antiretroviral drugs. Eighteen patients with CD4(+) T cell counts >/= 350 cells/microliter and viral load below the limits of detection for >/=1 year while on HAART were enrolled prospectively in a trial in which HAART was discontinued. Twelve of these patients had received prior IL-2 therapy and had low frequencies of resting, latently infected CD4 cells. Viral load relapse to >50 copies/ml occurred in all 18 patients independent of prior IL-2 treatment, beginning most commonly during weeks 2-3 after cessation of HAART. The mean relapse rate constant was 0.45 (0.20 log(10) copies) day(-1), which was very similar to the mean viral clearance rate constant after drug resumption of 0.35 (0.15 log(10) copies) day(-1) (P = 0.28). One patient experienced a relapse delay to week 7. All patients except one experienced a relapse burden to >5,000 RNA copies/ml. Ex vivo labeling with BrdUrd showed that CD4 and CD8 cell turnover increased after withdrawal of HAART and correlated with viral load whereas lymphocyte turnover decreased after reinitiation of drug treatment. Virologic relapse occurs rapidly in patients who discontinue suppressive drug therapy, even in patients with a markedly diminished pool of resting, latently infected CD4(+) T cells.
Figures
Figure 1
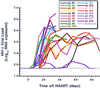
Three representative graphs of viral relapse after discontinuation of HAART, with linear regression lines (red) depicting back extrapolation of viral load to time zero (HAART withdrawal). bDNA results of <50 copies/ml were assigned values of 49 copies/ml before log10 transformation. Vertical black lines indicate when HAART was restarted.
Figure 2
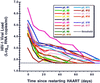
The viral relapse patterns observed in 18 patients in whom HAART was discontinued, color-coded as indicated.
Figure 3
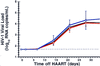
The mean viral load results (± SE) for the 18 patients (black line), separated into the 12 previous IL-2-recipients (red line) and the 6 non-IL-2 recipients (blue line).
Figure 4
The individual patterns of viral load suppression in patients after resumption of HAART, normalized to time zero (the day HAART was restarted in each patient), color-coded as in Fig. 2.
Figure 5
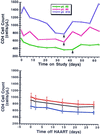
Changes in absolute CD4 cell counts experienced by patients in whom HAART was discontinued. (Upper) CD4 cell changes in three representative patients over time after discontinuation and then resumption of HAART. Vertical black arrows indicate when HAART was restarted in each patient. (Lower) The mean CD4 cell counts (± SE) for the 18 patients (black line) after discontinuation of HAART, separated into the 12 previous IL-2-recipients (red line) and the 6 non-IL-2 recipients (blue line).
Figure 6
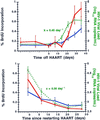
Changes in lymphocyte turnover, as measured by percent incorporation of BrdUrd, in relationship to plasma viral load before and after interruption of HAART. (Upper) Mean percent BrdUrd incorporation (±SE) into CD4+ (solid blue line) and CD8+ (solid red line) T cells at baseline and then after stopping HAART in comparison to plasma viral load (green dashed line). The mean viral relapse rate constant k is indicated. (Lower) Similar mean changes in CD4+ (solid blue line) and CD8+ (solid red line) T cells after reinitiating HAART therapy, normalized to time zero (the day HAART was restarted in each patient) and shown in comparison to the plasma viral load (green dashed line). The mean viral clearance rate constant c is indicated.
Figure 7
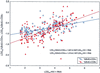
The correlation between log10 plasma RNA HIV-1 RNA levels and logarithmic percent BrdUrd incorporation into both CD4+ and CD8+ T cells (P < 0.0001). Only time points at which plasma viral RNA was in the detectable range after withdrawal of therapy are plotted.
Similar articles
- Inferiority of IL-2 alone versus IL-2 with HAART in maintaining CD4 T cell counts during HAART interruption: a randomized controlled trial.
Porter BO, Anthony KB, Shen J, Hahn B, Keh CE, Maldarelli F, Blackwelder WC, Lane HC, Kovacs JA, Davey RT, Sereti I. Porter BO, et al. AIDS. 2009 Jan 14;23(2):203-12. doi: 10.1097/QAD.0b013e32831cc114. AIDS. 2009. PMID: 19098490 Free PMC article. Clinical Trial. - A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection.
Hecht FM, Wang L, Collier A, Little S, Markowitz M, Margolick J, Kilby JM, Daar E, Conway B, Holte S; AIEDRP Network. Hecht FM, et al. J Infect Dis. 2006 Sep 15;194(6):725-33. doi: 10.1086/506616. Epub 2006 Aug 15. J Infect Dis. 2006. PMID: 16941337 Clinical Trial. - Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: A randomized controlled trial.
Davey RT Jr, Murphy RL, Graziano FM, Boswell SL, Pavia AT, Cancio M, Nadler JP, Chaitt DG, Dewar RL, Sahner DK, Duliege AM, Capra WB, Leong WP, Giedlin MA, Lane HC, Kahn JO. Davey RT Jr, et al. JAMA. 2000 Jul 12;284(2):183-9. doi: 10.1001/jama.284.2.183. JAMA. 2000. PMID: 10889591 Clinical Trial. - Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.
Van Vaerenbergh K. Van Vaerenbergh K. Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review. - The impact of highly active antiretroviral therapy on HIV-specific immune function.
Saag MS. Saag MS. AIDS. 2001 Feb;15 Suppl 2:S4-10. doi: 10.1097/00002030-200102002-00002. AIDS. 2001. PMID: 11424976 Review.
Cited by
- HIV vaccination: Navigating the path to a transformative breakthrough-A review of current evidence.
Scott GY, Worku D. Scott GY, et al. Health Sci Rep. 2024 Sep 23;7(9):e70089. doi: 10.1002/hsr2.70089. eCollection 2024 Sep. Health Sci Rep. 2024. PMID: 39319247 Free PMC article. - Integrator complex subunit 12 knockout overcomes a transcriptional block to HIV latency reversal.
Gray CN, Ashokkumar M, Janssens DH, Kirchherr J, Allard B, Hsieh E, Hafer TL, Archin NM, Browne EP, Emerman M. Gray CN, et al. bioRxiv [Preprint]. 2024 Sep 28:2024.08.30.610517. doi: 10.1101/2024.08.30.610517. bioRxiv. 2024. PMID: 39257755 Free PMC article. Preprint. - Administration of anti-HIV-1 broadly neutralizing monoclonal antibodies with increased affinity to Fcγ receptors during acute SHIVAD8-EO infection.
Dias J, Fabozzi G, Fourati S, Chen X, Liu C, Ambrozak DR, Ransier A, Laboune F, Hu J, Shi W, March K, Maximova AA, Schmidt SD, Samsel J, Talana CA, Ernste K, Ko SH, Lucas ME, Radecki PE, Boswell KL, Nishimura Y, Todd JP, Martin MA, Petrovas C, Boritz EA, Doria-Rose NA, Douek DC, Sékaly RP, Lifson JD, Asokan M, Gama L, Mascola JR, Pegu A, Koup RA. Dias J, et al. Nat Commun. 2024 Aug 29;15(1):7461. doi: 10.1038/s41467-024-51848-y. Nat Commun. 2024. PMID: 39198422 Free PMC article. - Engineered deletions of HIV replicate conditionally to reduce disease in nonhuman primates.
Pitchai FNN, Tanner EJ, Khetan N, Vasen G, Levrel C, Kumar AJ, Pandey S, Ordonez T, Barnette P, Spencer D, Jung SY, Glazier J, Thompson C, Harvey-Vera A, Son HI, Son HI, Strathdee SA, Holguin L, Urak R, Burnett J, Burgess W, Busman-Sahay K, Estes JD, Hessell A, Fennessey CM, Keele BF, Haigwood NL, Weinberger LS. Pitchai FNN, et al. Science. 2024 Aug 9;385(6709):eadn5866. doi: 10.1126/science.adn5866. Epub 2024 Aug 9. Science. 2024. PMID: 39116226 - ORC1 enhances repressive epigenetic modifications on HIV-1 LTR to promote HIV-1 latency.
Zhou M, Yang T, Yuan M, Li X, Deng J, Wu S, Zhong Z, Lin Y, Zhang W, Xia B, Wu Y, Wang L, Chen T, Liu R, Pan T, Ma X, Li L, Liu B, Zhang H. Zhou M, et al. J Virol. 2024 Aug 20;98(8):e0003524. doi: 10.1128/jvi.00035-24. Epub 2024 Jul 31. J Virol. 2024. PMID: 39082875
References
- Chiasson M A, Berenson L, Li W H, Schwartz S, Singh T, Forlenza S, Mojica B A, Hamburg M A. J Acquired Immune Defic Syndr Hum Retrovirol. 1999;21:59–64. - PubMed
- Powderly W G, Landay A, Lederman M M. J Am Med Assoc. 1998;280:72–77. - PubMed
- Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm D J, Cooper D A. AIDS. 1998;12:F51–F58. - PubMed
- Miller K D, Jones E, Yanovski J A, Shankar R, Feuerstein I, Falloon J. Lancet. 1998;351:871–875. - PubMed
- Carr A, Samaras K, Chisholm D J, Cooper D A. Lancet. 1998;351:1881–1883. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials