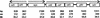Regulation of the alpha-galactosidase activity in Streptococcus pneumoniae: characterization of the raffinose utilization system - PubMed (original) (raw)
Regulation of the alpha-galactosidase activity in Streptococcus pneumoniae: characterization of the raffinose utilization system
C Rosenow et al. Genome Res. 1999 Dec.
Abstract
A 10.2-kb gene region was identified in the Streptococcus pneumoniae genome sequence that contains eight genes involved in regulation and metabolism of raffinose. The genes rafR and rafS are transcribed as one operon, and their gene products regulate the raffinose-dependent stimulation of a divergently transcribed second promoter (P(A)) directing the expression of aga, the structural gene for alpha-galactosidase. Raffinose-mediated transcription from P(A) results in a 500-fold increase in alpha-galactosidase activity in the cell. A third promoter within the cluster is responsible for the transcription of the remaining five genes (rafE, rafF, rafG, gtfA, and rafX), whose gene products might be involved in transport and metabolism of raffinose. The presence of additional internal promoters cannot be excluded. The aga promoter P(A) is negatively regulated by the presence of sucrose in the growth medium. Consistent with catabolite repression (CR), a DNA sequence with high homology to the CRE (cis-active element) was identified upstream of the aga promoter. Sucrose-mediated CR depends on the phosphoenolpyruvate: sucrose phosphotransferase system (PTS) but is unaffected by a mutation in a gene encoding a homolog of the CRE regulatory protein CcpA.
Figures
Figure 1
Map of the raf genes in the S. pneumoniae contig. Arrows indicate operons and the orientation of transcription. The number of base pairs (bp), amino acids (aa), and the molecular mass (_M_r) of the corresponding proteins are indicated.
Figure 2
Sequence analysis of the intergenic region between rafR and aga (A) and aga and rafE (B). Potential ribosome binding sites (RBS), −10 and −35 promoter elements (italics), and the direct repeats (arrows) are indicated. A 15-bp sequence with homology to the CRE consensus sequence is underlined.
Figure 3
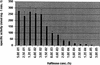
α-Galactosidase activities in the cell lysates of S. pneumoniae strain R6x grown in the presence of 0.2% glucose and different raffinose concentrations in the growth medium. The activities are expressed in units (nmoles of _p_-nitrophenol produced per min of incubation at room temperature) per mg protein with their
s.d.
.
Figure 4
Amplified cDNA from total RNA from the S. pneumoniae strain R6x grown in C+Y supplemented with 0.2% raffinose and 0.2% glucose. In each reaction (lanes 1_–_10) one primer pair was used, as indicated by the lines. The expected size for each fragment is given in kb. Lane 11 is a negative control (−) without RT and the primer pair from lane 3 in the reaction mixture; Lane 12 is a positive control (+) with total RNA and primers provided with the Access RT–PCR introductory System. The arrows indicate the identified potential promoters.
Similar articles
- Role of dihydrolipoamide dehydrogenase in regulation of raffinose transport in Streptococcus pneumoniae.
Tyx RE, Roche-Hakansson H, Hakansson AP. Tyx RE, et al. J Bacteriol. 2011 Jul;193(14):3512-24. doi: 10.1128/JB.01410-10. Epub 2011 May 20. J Bacteriol. 2011. PMID: 21602335 Free PMC article. - The melREDCA Operon Encodes a Utilization System for the Raffinose Family of Oligosaccharides in Bacillus subtilis.
Morabbi Heravi K, Watzlawick H, Altenbuchner J. Morabbi Heravi K, et al. J Bacteriol. 2019 Jul 10;201(15):e00109-19. doi: 10.1128/JB.00109-19. Print 2019 Aug 1. J Bacteriol. 2019. PMID: 31138628 Free PMC article. - Molecular analysis of an enigmatic Streptococcus pneumoniae virulence factor: The raffinose-family oligosaccharide utilization system.
Hobbs JK, Meier EPW, Pluvinage B, Mey MA, Boraston AB. Hobbs JK, et al. J Biol Chem. 2019 Nov 15;294(46):17197-17208. doi: 10.1074/jbc.RA119.010280. Epub 2019 Oct 7. J Biol Chem. 2019. PMID: 31591266 Free PMC article. - Nucleotide sequences and operon structure of plasmid-borne genes mediating uptake and utilization of raffinose in Escherichia coli.
Aslanidis C, Schmid K, Schmitt R. Aslanidis C, et al. J Bacteriol. 1989 Dec;171(12):6753-63. doi: 10.1128/jb.171.12.6753-6763.1989. J Bacteriol. 1989. PMID: 2556373 Free PMC article.
Cited by
- Polyamine Synthesis Effects Capsule Expression by Reduction of Precursors in Streptococcus pneumoniae.
Ayoola MB, Shack LA, Nakamya MF, Thornton JA, Swiatlo E, Nanduri B. Ayoola MB, et al. Front Microbiol. 2019 Aug 29;10:1996. doi: 10.3389/fmicb.2019.01996. eCollection 2019. Front Microbiol. 2019. PMID: 31555234 Free PMC article. - A novel protein, RafX, is important for common cell wall polysaccharide biosynthesis in Streptococcus pneumoniae: implications for bacterial virulence.
Wu K, Huang J, Zhang Y, Xu W, Xu H, Wang L, Cao J, Zhang X, Yin Y. Wu K, et al. J Bacteriol. 2014 Sep;196(18):3324-34. doi: 10.1128/JB.01696-14. Epub 2014 Jul 7. J Bacteriol. 2014. PMID: 25002545 Free PMC article. - Bacterial surface lipoproteins mediate epithelial microinvasion by Streptococcus pneumoniae.
Chan JM, Ramos-Sevillano E, Betts M, Wilson HU, Weight CM, Houhou-Ousalah A, Pollara G, Brown JS, Heyderman RS. Chan JM, et al. Infect Immun. 2024 May 7;92(5):e0044723. doi: 10.1128/iai.00447-23. Epub 2024 Apr 17. Infect Immun. 2024. PMID: 38629841 Free PMC article. - Isolation and Identification of an α-Galactosidase-Producing Lactosphaera pasteurii Strain and Its Enzymatic Expression Analysis.
Zhao Y, Zhou J, Dai S, Liu X, Zhang X. Zhao Y, et al. Molecules. 2022 Sep 13;27(18):5942. doi: 10.3390/molecules27185942. Molecules. 2022. PMID: 36144675 Free PMC article. - Cellobiose-mediated gene expression in Streptococcus pneumoniae: a repressor function of the novel GntR-type regulator BguR.
Shafeeq S, Kuipers OP, Kloosterman TG. Shafeeq S, et al. PLoS One. 2013;8(2):e57586. doi: 10.1371/journal.pone.0057586. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23469031 Free PMC article.
References
- Busque P, Letellier A, Harel J, Dubreuil JD. Production of Escherichia coli STb enterotoxin is subject to catabolite repression. Microbiology. 1995;141:1621–1627. - PubMed
- Claverys JP, Dintilhac A, Pestova EV, Martin B, Morrison DA. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene. 1995;164:123–128. - PubMed
- Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolitic activity to carbon catabolite repression in Gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous