Caspase-9 and APAF-1 form an active holoenzyme - PubMed (original) (raw)
Caspase-9 and APAF-1 form an active holoenzyme
J Rodriguez et al. Genes Dev. 1999.
Abstract
Autocatalytic activation of initiator caspases is the link between pro-apoptotic signals and the destruction machinery of apoptosis. Activation of caspase-9, which mediates oncogene and drug-induced apoptosis, requires binding to the protein APAF-1. We found that the proteolytic activity of caspase-9 in a complex with APAF-1 is several orders of magnitude higher than that of the free enzyme. Thus, this complex functions as a holoenzyme in which caspase-9 is the catalytic subunit and APAF-1 its allosteric regulator. We argue that caspase-9 is activated by allosteric regulation and suggest that this mechanism is common for other initiator caspases.
Figures
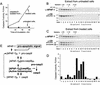
Figure 1
The rate of caspase-3 activation correlates with the amount of APAF-1 bound to caspase-9 (A,B) Caspases in 293 extract were activated by adding 5 m
m
ATP or dATP followed by incubation at 37°C. At the indicated times, caspase-3 activity using DEVD-AFC as a substrate (A) and caspase-3 processing, by immunoblotting with antibodies as indicated was assayed (B). (C,D) At the indicated times aliquots of the extracts were used to immunoprecipitate caspase-9. Caspase-9 (C) and APAF-1 (D) in the precipitate were detected by immunoblotting. (E,F) A fraction of processed caspase-9 is in a putative complex with APAF-1 (star in F). A total of 50 μl (1.5 mg) of 293 extract was supplemented with 5 m
m
dATP and incubated at 37°C for 8 min (active extract). A control aliquot was incubated with buffer (inactive extract). Both aliquots were fractionated on 5 ml of 5%–20% linear sucrose gradients and the fractions were analyzed by immunoblotting for APAF-1, caspase-9, and cytochrome c. Sedimentation markers have been described.
Figure 2
Caspase-9 is active only in a complex with APAF-1. (A) Caspase-9 depleted from active extracts can activate caspase-3. Caspase-9 was depleted from active or inactive extracts eluted with the epitope peptide, and its activity was analyzed using caspase-3 precursor as a substrate. Sepharose with no antibody bound was used for mock depletions. (B) Caspase-9 depleted from active extracts can process catalytically inactive caspase-3. The experiment was done as in A, but the caspase-3 used was made inactive by mutating the catalytic Cys-163 to serine. (C,D) Caspase-9 was depleted from 4 ml (∼135 mg) active extract as in A and fractionated, analyzed for APAF-1 and caspase-9 by immunoblotting (C) and for caspase-9 activity (D) using caspase-3 as in B (1-hr incubation). APAF-1 and caspase-9 in fraction 17 (the bottom of the gradient) are likely to be an aggregate formed by anti-caspase-9 antibodies that leached from Sepharose and were detected only in this fraction (not shown). (E,F) Both free processed caspase-9 and the caspase-9 holoenzyme can rescue caspase-3 activation in extracts depleted of caspase-9. A total of 240 μl (6.4 mg) of active or inactive extracts was fractionated by sedimentation in sucrose gradients and caspase-9 was immunoprecipitated from each fraction and eluted with the epitope peptide. The amounts of caspase-9 and APAF-1 that coprecipitated with caspase-9 from the active extract were estimated in the eluates by immunoblotting (E,F). The eluates were added to aliquots of inactive extract that was immunodepleted of caspase-9. The aliquots were then incubated for 15 min at 37°C either with buffer (G,H) or dATP (I,J) and caspase-3 activity was measured with DEVD–AFC as a substrate.
Figure 3
(A–C) Binding to APAF-1 increases the catalytic activity of caspase-9. A total of 1.5 ml (60 mg) of active extract was fractionated by sedimentation. Each fraction was divided into two aliquots, one of which was immunoprecipitated with anti-caspase-9–Sepharose, and the other mock precipitated with Sepharose. Both precipitates were eluted with the epitope peptide. (A) The amount of caspase-9 and APAF-1 in the anti-caspase-9–Sepharose eluate as revealed by immunoblotting. The activity of the eluates [(solid bars) anti-caspase-9, (open bars) mock] was measured using either caspase-3 precursor (B) or a IETD–AFC peptide substrate (C). The open bars in B are not seen because the activity of the mock precipitates was low. (D) The main components of the holoenzyme are APAF-1 and caspase-9. Catalytically inactive caspase-9 containing the Flag epitope tag was stably expressed in 293 cells. A total of 500 μl (20 mg) of extract from these cells was incubated with 1 m
m
dATP at 37°C for 10 min, and caspase-9 was precipitated using anti-Flag antibodies linked to agarose (Sigma). An equal amount of extract from parental 293 cells was treated identically and used for a control precipitation. The precipitates from both extracts were fractionated on a 10% or 15% (not shown) SDS–polyacrylamide gel, which was then stained with Coomassie blue. Caspase-9 and APAF-1 were identified by immunoblotting and immunoprecipitation (data not shown).
Figure 4
Caspase-9 is active in cells as a holoenzyme. Human fibroblasts transformed with the adenoviral E1A oncogene (IMR90-E1A) were either treated with 50 μ
m
etoposide for 18 hr (sixteen 15-cm plates) or left untreated (8 plates) and used to prepare extracts (Liu et al. 1996). (A) Only caspase-9 from treated cells is active. Caspase-9 was precipitated from the extracts and assayed for activity using caspase-3. (B,C) A total of 600 μl (9 mg) of each extract was fractionated by sedimentation in sucrose gradients, and the amount of caspase-9 and APAF-1 in the fractions evaluated by immunoblotting. (D) Caspase-9 was immunoprecipitated from the fractions, eluted with the epitope peptide, and the activity was measured as in A (14 hr incubation). (E) A model for caspase-9 activation and activity.
Similar articles
- Chemical-induced apoptosis: formation of the Apaf-1 apoptosome.
Cain K. Cain K. Drug Metab Rev. 2003 Nov;35(4):337-63. doi: 10.1081/dmr-120026497. Drug Metab Rev. 2003. PMID: 14705865 Review. - Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome.
Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Beere HM, et al. Nat Cell Biol. 2000 Aug;2(8):469-75. doi: 10.1038/35019501. Nat Cell Biol. 2000. PMID: 10934466 - Oligomerization and activation of caspase-9, induced by Apaf-1 CARD.
Shiozaki EN, Chai J, Shi Y. Shiozaki EN, et al. Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4197-202. doi: 10.1073/pnas.072544399. Epub 2002 Mar 19. Proc Natl Acad Sci U S A. 2002. PMID: 11904389 Free PMC article. - Oligomerization is a general mechanism for the activation of apoptosis initiator and inflammatory procaspases.
Chang DW, Ditsworth D, Liu H, Srinivasula SM, Alnemri ES, Yang X. Chang DW, et al. J Biol Chem. 2003 May 9;278(19):16466-9. doi: 10.1074/jbc.C300089200. Epub 2003 Mar 13. J Biol Chem. 2003. PMID: 12637514 - Apoptosome: the cellular engine for the activation of caspase-9.
Shi Y. Shi Y. Structure. 2002 Mar;10(3):285-8. doi: 10.1016/s0969-2126(02)00732-3. Structure. 2002. PMID: 12005427 Review.
Cited by
- Caspase control: protagonists of cancer cell apoptosis.
Fiandalo MV, Kyprianou N. Fiandalo MV, et al. Exp Oncol. 2012 Oct;34(3):165-75. Exp Oncol. 2012. PMID: 23070001 Free PMC article. Review. - Regulation of the intrinsic apoptosis pathway by reactive oxygen species.
Wu CC, Bratton SB. Wu CC, et al. Antioxid Redox Signal. 2013 Aug 20;19(6):546-58. doi: 10.1089/ars.2012.4905. Epub 2012 Oct 25. Antioxid Redox Signal. 2013. PMID: 22978471 Free PMC article. Review. - Live to die another way: modes of programmed cell death and the signals emanating from dying cells.
Fuchs Y, Steller H. Fuchs Y, et al. Nat Rev Mol Cell Biol. 2015 Jun;16(6):329-44. doi: 10.1038/nrm3999. Epub 2015 May 20. Nat Rev Mol Cell Biol. 2015. PMID: 25991373 Free PMC article. Review. - The structure of procaspase 6 is similar to that of active mature caspase 6.
Kang BH, Ko E, Kwon OK, Choi KY. Kang BH, et al. Biochem J. 2002 Jun 15;364(Pt 3):629-34. doi: 10.1042/BJ20011787. Biochem J. 2002. PMID: 12049625 Free PMC article.
References
- Cain K, Brown DG, Langlais C, Cohen GM. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J Biol Chem. 1999;274:22686–22692. - PubMed
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. - PubMed
- Imai Y, Kimura T, Murakami A, Yajima N, Sakamaki K, Yonehara S. The CED-4 homologous protein FLASH is involved in Fas-mediated activation of caspase-8 during apoptosis. Nature. 1999;398:777–785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases