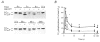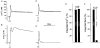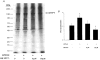Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum - PubMed (original) (raw)
Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum
K Swärd et al. J Physiol. 2000.
Abstract
Ca2+ sensitization of smooth muscle contraction involves the small GTPase RhoA, inhibition of myosin light chain phosphatase (MLCP) and enhanced myosin regulatory light chain (LC20) phosphorylation. A potential effector of RhoA is Rho-associated kinase (ROK). The role of ROK in Ca2+ sensitization was investigated in guinea-pig ileum. Contraction of permeabilized muscle strips induced by GTPgammaS at pCa 6.5 was inhibited by the kinase inhibitors Y-27632, HA1077 and H-7 with IC50 values that correlated with the known Ki values for inhibition of ROK. GTPgammaS also increased LC20 phosphorylation and this was prevented by HA1077. Contraction and LC20 phosphorylation elicited at pCa 5.75 were, however, unaffected by HA1077. Pre-treatment of intact tissue strips with HA1077 abolished the tonic component of carbachol-induced contraction and the sustained elevation of LC20 phosphorylation, but had no effect on the transient or sustained increase in [Ca2+]i induced by carbachol. LC20 phosphorylation and contraction dynamics suggest that the ROK-mediated increase in LC20 phosphorylation is due to MLCP inhibition, not myosin light chain kinase activation. In the absence of Ca2+, GTPgammaS stimulated 35S incorporation from [35S]ATPgammaS into the myosin targeting subunit of MLCP (MYPT). The enhanced thiophosphorylation was inhibited by HA1077. No thiophosphorylation of LC20 was detected. These results indicate that ROK mediates agonist-induced increases in myosin phosphorylation and force by inhibiting MLCP activity through phosphorylation of MYPT. Under Ca2+-free conditions, ROK does not appear to phosphorylate LC20 in situ, in contrast to its ability to phosphorylate myosin in vitro. In particular, ROK activation is essential for the tonic phase of agonist-induced contraction.
Figures
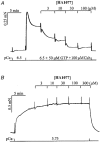
Figure 8. Effect of HA1077 on carbachol-induced LC20 phosphorylation in intact guinea-pig ileum
Intact guinea-pig ileum longitudinal smooth muscle strips were treated with carbachol (100 μM) in the absence and presence of HA1077 (6 or 30 μM) for the indicated times at which the tissue was snap-frozen and LC20 phosphorylation quantified as described in Methods. A, representative Western blots of LC20 after separation of the phosphorylated from the unphosphorylated species by urea-glycerol gel electrophoresis. B, quantitative data pooled from several experiments (n = 6–16). Control (•), 6 μM HA1077 (▿), 30 μM HA1077 (□). *Significant differences from LC20 phosphorylation levels at time zero.
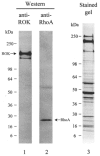
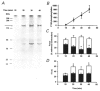
Figure 1. Identification of ROK and RhoA in guinea-pig ileum longitudinal smooth muscle
Tissue homogenates were subjected to SDS-PAGE and either stained with Coomassie Brilliant Blue (lane 3) or immunoblotted with anti-ROKβ (lane 1) or anti-RhoA (lane 2). The equivalent of 0.625 mg wet weight of tissue was applied to lane 1, 2.5 mg wet weight of tissue to lane 2 and 2.5 mg wet weight of tissue to lane 3. Results are representative of three independent experiments.
Figure 2. Effects of kinase inhibitors on Ca2+ sensitization of force induced in permeabilized guinea-pig ileum by GTPγS
A, guinea-pig ileum longitudinal smooth muscle permeabilized with β-escin was contracted by increasing [Ca2+] from pCa 9 to 4.5 and relaxed by returning to pCa 9. Transfer to pCa 6.5 solution failed to elicit a significant contraction but subsequent transfer to pCa 6.5 + GTPγS (100 μM) evoked Ca2+ sensitization of force. The effects of increasing concentrations of three kinase inhibitors (Y-27632, HA1077 and H-7) on Ca2+ sensitization were recorded. B, correlation between IC50 values for inhibition of Ca2+ sensitization and the known _K_i values for inhibition of ROK (n = 5).
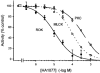
Figure 3. Sensitivity of ROK, MLCK and PKC to the kinase inhibitor HA1077
The effects of increasing concentrations of HA1077 on ROK (•), MLCK (○) and PKC activities (□) were determined as described in Methods. Activity in the presence of vehicle was expressed as 100 %.
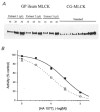
Figure 4. Quantification of MLCK levels in guinea-pig ileum and the effect of [ATP] on inhibition of MLCK by HA1077
A, guinea-pig ileum longitudinal smooth muscle proteins were extracted as described in Methods, subjected to SDS-PAGE and immunoblotted with anti-MLCK. Three loading levels of three independent extracts and different known amounts of purified chicken gizzard MLCK (0.05, 0.1, 0.15, 0.2, 0.4 and 0.8 μg) were applied to the gel. MLCK bands were quantified by densitometric scanning. A standard curve relating the intensity of the chicken gizzard MLCK bands (optical density × area of the band) to micrograms of MLCK loaded was linear with an _r_2 value of 0.98. B, the effect of increasing concentrations of HA1077 on the activity of purified MLCK was assayed as described in Methods at 0.25 (○) and 1 mM ATP (•).
Figure 5. Effects of HA1077 on Ca2+ sensitization of force evoked by agonist plus GTP and Ca2+-induced contraction
A, force in β-escin-permeabilized guinea-pig ileum longitudinal smooth muscle was activated at a sub-threshold [Ca2+] (pCa 6.5) by addition of 100 μM carbachol (Cch) and 50 μM GTP. Cumulative addition of HA1077 inhibited this Ca2+ sensitization. In control strips (n = 6), Cch + GTP-induced steady-state force at pCa 6.5 was maintained for at least 40 min. Ca2+ sensitization induced by Cch + GTP was inhibited by 100 μM atropine, verifying that the Cch effect was mediated by muscarinic receptors. B, force was maximally activated at pCa 5.75 and was unaffected by cumulative addition of HA1077 up to 300 μM.
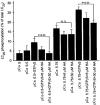
Figure 6. Effect of HA1077 (HA) on the GTPγS-induced increase in LC20 phosphorylation in permeabilized guinea-pig ileum
β-Escin-permeabilized guinea-pig ileum smooth muscle strips were exposed to the indicated conditions for 5 min, following which the strips were snap-frozen and LC20 phosphorylation levels quantified as described in Methods. Columns and bars represent the means and
s.e.m.
s. Columns 1–5, n = 5; column 6, n = 11; columns 7–11, n = 6. N.S., not significant.
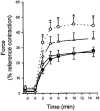
Figure 7. Effects of HA1077 on carbachol-induced changes in [Ca2+]i and force in intact guinea-pig ileum
Intact guinea-pig ileum longitudinal smooth muscle strips were loaded with fura-2 AM. [Ca2+]i (A and C) and force (B and D) were recorded following stimulation with carbachol (100 μM) in the absence (A and B) and presence (C and D) of HA1077 (30 μM). HA1077 was present 4 min prior to, and during, the carbachol-induced contraction. Integrated and normalized [Ca2+]i (E) and integrated and normalized force (F) were recorded in controls and after exposure to HA1077 (n = 7).
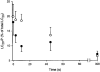
Figure 9. Effect of HA1077 on the rate of LC20 dephosphorylation in permeabilized guinea-pig ileum
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were maximally contracted at pCa 4.5 in the presence of GTPγS (100 μM). HA1077 (30 μM, •) or vehicle (control, ○) was added 5–10 min prior to relaxation which was initiated at time zero by transfer to rigor solution at pCa 9 containing GTPγS (100 μM). Tissues were snap-frozen at the indicated times for quantification of LC20 phosphorylation levels (LC20-P) as described in Methods.
Figure 10. Thiophosphorylation of a 130 kDa protein correlates with Ca2+ sensitization of contraction
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were incubated with 100 μM ML-9 in pCa 9 rigor solution and 10 μM [35S]ATPγS was added at time zero. Muscle strips were snap-frozen at the indicated times and proteins extracted and separated by SDS-PAGE as described in Methods. The gels were stained with Coomassie Blue and thiophosphorylated proteins detected by autoradiography. A, a representative autoradiogram. The 130 kDa thiophosphorylated protein (identified as MYPT by Western blotting) is indicated by the arrow. The positions of molecular weight markers are indicated to the left. Note the absence of LC20 thiophosphorylation. B, cumulative data (n = 3) showing the time course of MYPT thiophosphorylation. Values were normalized to the Coomassie Blue-stained calponin band to correct for variations in protein loading levels. C, to evaluate the mechanical effect of MYPT thiophosphorylation, permeabilized muscle strips were incubated in the absence (▪) or presence of ATPγS (□) as described above. Tissues were then washed in pCa 9 solution (3 × 5 min) and contracted in pCa 6.0 solution. *Significant difference (P < 0.05) from 10 min controls (_n_ >= 10). D, after contraction in response to immersion in pCa 4.5 solution, relaxation was recorded at high chart speed to obtain _t_½ values for relaxation. *Significant difference from the 10 min plus ATPγS value. In C and D, values recorded in the presence of ATPγS differ significantly from controls at all times.
Figure 11. GTPγS enhances MYPT thiophosphorylation
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were incubated in pCa 9 rigor solution containing 10 μM [35S]ATPγS in the absence or presence of 1 μM GTPγS. Muscle strips were processed as described in the legend to Fig. 10. A, a representative autoradiogram. Numbers below each lane indicate the radioactivity in the 130 kDa band normalized to the 10 min control value. B, a portion of the Coomassie Blue-stained gel to show the filamin band that was used to correct for variations in protein loading levels. Numbers below the gel lanes denote the optical density × area of the filamin band in each lane. C, cumulative data (n >= 5) showing the time course of MYPT thiophosphorylation in the absence (shaded bars) and presence (open bars) of GTPγS. Values are corrected for background and protein loading and are normalized to the control (no GTPγS) value at 10 min. D, permeabilized muscle strips were incubated in pCa 9 rigor solution containing 100 μM ML-9 in the absence (shaded bars; n = 4) or presence (open bars; n = 4) of 10 μM ATPγS. After washing, muscle strips were contracted in pCa 6.25 solution. At the ensuing plateau, 100 μM GTPγS was added. Finally, muscle strips were contracted in pCa 4.5 solution.
Figure 12. Addition of carbachol and GTP during ATPγS pre-incubation enhances the subsequent response to Ca2+ which is blocked by ROK inhibition
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were pre-incubated in pCa 9 rigor solution containing 10 μM ATPγS with the following additions: none (•); 100 μM carbachol and 10 μM GTP (○); 100 μM carbachol, 10 μM GTP and 30 μM Y-27632 (▿); or 100 μM carbachol, 10 μM GTP and 30 μM HA1077 (▵). Muscle strips were then washed in pCa 9 solution containing MgATP to ensure washout of acute sensitization by carbachol plus GTP. Force development upon re-addition of MgATP was not significant in either condition but differed during the subsequent pCa 6.25-induced contraction (initiated at time zero). *Significant difference compared with control (ATPγS alone).
Figure 13. Inhibition of the GTPγS-induced increase in MYPT thiophosphorylation by ROK inhibition
β-Escin-permeabilized guinea-pig ileum longitudinal smooth muscle strips were incubated in pCa 9 rigor solution. At time zero, 10 μM [35S]ATPγS was added without or with 1 μM GTPγS in the absence or presence of HA1077 (6 or 30 μM) as indicated. Muscle strips were processed after 10 min as described in the legend to Fig. 10. A, a representative autoradiogram. B, cumulative data (n = 8) showing the GTPγS-induced increase in MYPT thiophosphorylation and its prevention by HA1077. *Significant difference from control. †Significant difference from value measured in the presence of GTPγS alone. More thiophosphorylated bands are visible in A than in Fig. 11_A_ because the gel was exposed to the X-ray film for a longer period of time.
Similar articles
- Thiophosphorylation-induced Ca(2+) sensitization of guinea-pig ileum contractility is not mediated by Rho-associated kinase.
Pfitzer G, Sonntag-Bensch D, Brkic-Koric D. Pfitzer G, et al. J Physiol. 2001 Jun 15;533(Pt 3):651-64. doi: 10.1111/j.1469-7793.2001.t01-2-00651.x. J Physiol. 2001. PMID: 11410624 Free PMC article. - ERK and p38MAPK pathways regulate myosin light chain phosphatase and contribute to Ca2+ sensitization of intestinal smooth muscle contraction.
Ihara E, Yu Q, Chappellaz M, MacDonald JA. Ihara E, et al. Neurogastroenterol Motil. 2015 Jan;27(1):135-46. doi: 10.1111/nmo.12491. Neurogastroenterol Motil. 2015. PMID: 25557225 - Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments.
Weber LP, Van Lierop JE, Walsh MP. Weber LP, et al. J Physiol. 1999 May 1;516 ( Pt 3)(Pt 3):805-24. doi: 10.1111/j.1469-7793.1999.0805u.x. J Physiol. 1999. PMID: 10200427 Free PMC article. - The regulation of smooth muscle contractility by zipper-interacting protein kinase.
Ihara E, MacDonald JA. Ihara E, et al. Can J Physiol Pharmacol. 2007 Jan;85(1):79-87. doi: 10.1139/y06-103. Can J Physiol Pharmacol. 2007. PMID: 17487247 Review. - The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction.
Swärd K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. Swärd K, et al. Curr Hypertens Rep. 2003 Feb;5(1):66-72. doi: 10.1007/s11906-003-0013-1. Curr Hypertens Rep. 2003. PMID: 12530938 Review.
Cited by
- Rho-ROCK and Rac-PAK signaling pathways have opposing effects on the cell-to-cell spread of Marek's Disease Virus.
Richerioux N, Blondeau C, Wiedemann A, Rémy S, Vautherot JF, Denesvre C. Richerioux N, et al. PLoS One. 2012;7(8):e44072. doi: 10.1371/journal.pone.0044072. Epub 2012 Aug 27. PLoS One. 2012. PMID: 22952878 Free PMC article. - Mechanisms involved in carbachol-induced Ca(2+) sensitization of contractile elements in rat proximal and distal colon.
Takeuchi T, Kushida M, Hirayama N, Kitayama M, Fujita A, Hata F. Takeuchi T, et al. Br J Pharmacol. 2004 Jun;142(4):657-66. doi: 10.1038/sj.bjp.0705820. Epub 2004 May 24. Br J Pharmacol. 2004. PMID: 15159278 Free PMC article. - Inhibition of ROCK activity allows InlF-mediated invasion and increased virulence of Listeria monocytogenes.
Kirchner M, Higgins DE. Kirchner M, et al. Mol Microbiol. 2008 May;68(3):749-67. doi: 10.1111/j.1365-2958.2008.06188.x. Epub 2008 Mar 4. Mol Microbiol. 2008. PMID: 18331468 Free PMC article. - Induction of oligodendrocyte differentiation and in vitro myelination by inhibition of rho-associated kinase.
Pedraza CE, Taylor C, Pereira A, Seng M, Tham CS, Izrael M, Webb M. Pedraza CE, et al. ASN Neuro. 2014 Jun 25;6(4):1759091414538134. doi: 10.1177/1759091414538134. ASN Neuro. 2014. PMID: 25289646 Free PMC article. - Involvement of Rho kinase and protein kinase C in carbachol-induced calcium sensitization in beta-escin skinned rat and guinea-pig bladders.
Durlu-Kandilci NT, Brading AF. Durlu-Kandilci NT, et al. Br J Pharmacol. 2006 Jun;148(3):376-84. doi: 10.1038/sj.bjp.0706723. Br J Pharmacol. 2006. PMID: 16565731 Free PMC article.
References
- Akopov SE, Zhang L, Pearce WJ. Regulation of Ca2+ sensitization by PKC and rho proteins in ovine cerebral arteries: effects of artery size and age. American Journal of Physiology. 1998;275:H930–939. - PubMed
- Allen BG, Andrea JE, Walsh MP. Identification and characterization of protein kinase C ζ-immunoreactive proteins. Journal of Biological Chemistry. 1994;269:29288–29298. - PubMed
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) Journal of Biological Chemistry. 1996;271:20246–20249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous