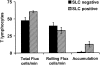The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules - PubMed (original) (raw)
The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules
R A Warnock et al. J Exp Med. 2000.
Abstract
Chemokines have been hypothesized to contribute to the selectivity of lymphocyte trafficking not only as chemoattractants, but also by triggering integrin-dependent sticking (arrest) of circulating lymphocytes at venular sites of extravasation. We show that T cells roll on most Peyer's patch high endothelial venules (PP-HEVs), but preferentially arrest in segments displaying high levels of luminal secondary lymphoid tissue chemokine (SLC) (6Ckine, Exodus-2, thymus-derived chemotactic agent 4 [TCA-4]). This arrest is selectively inhibited by functional deletion (desensitization) of CC chemokine receptor 7 (CCR7), the receptor for SLC and for macrophage inflammatory protein (MIP)-3beta (EBV-induced molecule 1 ligand chemokine [ELC]), and does not occur in mutant DDD/1 mice that are deficient in these CCR7 ligands. In contrast, pertussis toxin-sensitive B cell sticking does not require SLC or MIP-3beta signaling, and occurs efficiently in SLC(low/-) HEV segments in wild-type mice, and in the SLC-negative HEVs of DDD/1 mice. Remarkably, sites of T and B cell firm adhesion are segregated in PPs, with HEVs supporting B cell accumulation concentrated in or near follicles, the target domain of most B cells entering PPs, whereas T cells preferentially accumulate in interfollicular HEVs. Our findings reveal a fundamental difference in signaling requirements for PP-HEV recognition by T and B cells, and describe an unexpected level of specialization of HEVs that may allow differential, segmental control of lymphocyte subset recruitment into functionally distinct lymphoid microenvironments in vivo.
Figures
Figure 1
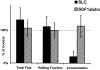
Desensitization of LNCs with SLC inhibits arrest in PP-HEVs. Unfractionated LNCs (70–85% CD3+ by FACS®) of young mice were washed and incubated in cRPMI for 1 h in tissue culture flasks. LNCs were fluorescently labeled, incubated with chemokine (SLC and SDF-1α) or control medium for 45 min at 37°C, washed through a warm serum gradient to remove residual chemokine, and resuspended together with internal standard cells (labeled in a different color) immediately before injection via carotid artery cannulation into mice prepared for intravital microscopy. Areas within the visualized PPs were surveyed in the initial 2 min for HEVs supporting lymphocyte interactions, and then observed for a further 10 min. Total flux and rolling data (for test and for internal standard cells) were collected during a 2-min period 6–8 min after injection, and represent at least 10 consecutive cells of each type in at least 10 HEVs per preparation. Accumulated cells in these same HEVs were enumerated at the 8-min time point. Lymphocyte total flux, rolling fraction, or accumulation was variable in different vessels and PPs, reflecting the natural variability in hemodynamic parameters and vascular characteristics. Therefore, values determined for experimental cells (chemokine-desensitized or mock-treated cells of one color) were normalized by dividing by values obtained for coinjected internal standard population (labeled in a separate color) determined in the same recipient vessels. The ratio of chemokine-treated cell to internal standard cell values (e.g., rolling fractions), determined in one set of recipients, was then divided by the ratio of mock-treated control cell to internal standard cell values determined in littermate recipients, and multiplied by 100. Thus, the data presented represent the total flux, rolling fraction, or accumulation of chemokine-treated cells expressed as a percentage of those of mock-treated control cells labeled identically and analyzed in parallel. Therefore, 100% in the graph represents the behavior of the mock-treated control cells: for these cells, the total flux of cells entering HEVs in the blood per minute ranged from 16 to 60, mean 36 ± 14 SD in a representative experiment; the rolling fraction ranged from 27 to 100%, mean 69 ± 20% SD; and the number of cells accumulated in HEV segments ranged from 0 to 60 or more by 10 min. These values illustrate the natural variability associated with different vascular segments and observation periods that necessitated the use of internal standard cells for these studies. Rolling velocity ranged from 25 to 170 μm/s, mean 81 ± 46 SD. Mean results of five experimental animals for each treatment (± SD) are shown in the graph. Inhibition of accumulation by SLC treatment is significant compared with SDF-1α or control treatment by Student's t test (P < 0.01).
Figure 3
Homologous but not heterologous desensitization by CCR7 and CXCR4 ligands. Desensitization of chemotaxis experiments were performed as described (reference 22). In brief, mouse LN or spleen cells were pretreated with five times the optimal chemotactic dose of the indicated chemokine (or control medium), and washed through a serum layer to remove residual chemokine. The desensitized cells were then allowed to migrate towards the same or another chemokine (at the optimal chemotactic dose) through 5-μm pores for 15 min. Migrated cells were counted as described (references 8, 22). Percent migration of B cells was calculated based on FACS® analysis of migrated compared with input cells; B cells were defined as CD3−/CD19+. Data shown are the mean ± SD of three experiments, each with two replicate wells per condition. Mean maximum migration of mock-treated control B cells to SDF-1α was 10%, to MIP-3β 15%, and to SLC 13%.
Figure 2
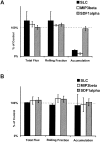
Effect of chemokine desensitization on T and B cell interactions with PP-HEVs. (A) T cell or (B) B cell subpopulations of LNCs were enriched by negative selection (see Materials and Methods), labeled, and incubated with 1 μM of indicated chemokine or control medium as in Fig. 1, then injected (mixed 1:1 with internal standard cells), and their behaviors were observed within PP-HEVs. Data collection was as in the legend to Fig. 1 except for B cells (which accumulated faster than T cells; data not shown), for which total flux and rolling fraction were analyzed during a 2-min time period 4–6 min after injection. Data are percentages of mock-treated control, and represent mean results of two to four experimental animals for each combination of subset and treatment (± SD). Control T cell total flux in vessels examined ranged from 14 to 60 cells/min, mean 48 ± 24 SD, and rolling fraction from ∼35 to 93%. Control B cell total flux ranged from 8 to 74 cells/min, mean 32 ± 18 SD, and rolling fraction from 25 to 84%, mean 54 ± 23%. Rolling velocity of T cells (range 23–197 μm/s, mean 94 ± 33 SD, comparable to previous reports [references 2, 41]) was not effected by SLC desensitization (range 24–160 μm/s, mean 99 ± 44 SD). B cell rolling velocities were similar (range 27–120 μm/s, mean 55 ± 25 SD). Inhibition of T cell accumulation by SLC or MIP-3β desensitization is significant compared with control or SDF-1α desensitization by Student's t test (P < 0.01).
Figure 5
SLC is expressed on the lumen of PP-HEVs in normal, but not DDD/1 mice. Confocal micrographs of whole PPs from normal (left and right panels) or DDD/1 (middle panel) mice depicting localization of green anti-SLC (left and middle panels), and lack of green staining with a negative control hamster antibody (right panel); and MAdCAM-1+ HEVs as red fluorescence (all three panels). mAbs to SLC (anti–TCA-4, 4B1E7C10 [reference 17]) and MAdCAM-1 (MECA 89), or control antibodies to DNP (UC8-19B) and human CD44 (Hermes-1) (not shown) were conjugated to Alexa™ 488 or Alexa™ 546, respectively. 50–75 μg of anti-SLC or anti-DNP, and/or 10–20 μg anti–MAdCAM-1 or anti-huCD44 was injected via carotid artery cannulation in a single bolus. Staining was evident for both SLC and MAdCAM-1 by 1 min, maximal for MAdCAM-1 by 10 min and for SLC by 15 min. Animals were killed, and whole PPs were immediately removed for examination by confocal microscopy. Representative images (10× objective).
Figure 4
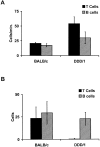
Interactions of T and B cells with PP-HEVs of DDD/1 versus normal mice. (A) The rolling flux of injected T cells (black bars) in PP-HEVs is increased in SLC-deficient DDD/1 mice compared with age-matched normal BALB/c, while B cell (hatched bars) rolling flux is comparable. (B) T cell accumulation is severely curtailed in PP-HEVs of DDD/1 mice; B cell accumulation is similar to control BALB/c mice. Enriched T and B cell (107 cells each) populations were differentially labeled and coinjected for observation in PP-HEVs. Venular trees draining single follicles were selected for analysis. Rolling flux is the total number of rolling B or T cells observed over time and averaged for the number of venules that supported rolling in the tree. Accumulation is the average number of stuck cells of each subset in the whole tree at 8 min after injection. Data are mean results of three experimental animals of each type (± SD). Rolling velocities of T cells in DDD/1 mice were similar to those in wild-type BALB/c mice (range 43–191 μm/s, mean 99 ± 44 SD).
Figure 6
T cells preferentially accumulate in SLC+ HEVs. T cells were observed in HEVs of normal animals as in the legend to Fig. 2. After observations of T cell behaviors in multiple fields, SLC+ HEVs were identified by subsequent, sequential injection of labeled anti-SLC and anti–MAdCAM-1. MAdCAM-1+ HEVs that exhibited successive segments differing only by expression of SLC were analyzed. Data are mean results of three experiments (± SD).
Figure 8
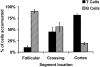
Sticking B and T cells are segregated in PP-HEVs. Accumulated B and T cells in PP-HEVs of normal mice were analyzed for preferential localization after injection of equal numbers of each subset. Follicles were identified by characteristic autofluorescence of resident dendritic cells and macrophages. HEV segments were defined as follicular if lying within or upon a follicle, cortical if wholly within the cortex, and crossing when transitional from follicle to cortex. Red (T) and green (B) accumulated cells were enumerated 8 min after injection in each class of vessels. Data are presented as percentage of B or T cells observed among cells in each vessel type; mean results of three experiments (± SD).
Figure 7
Localization of lymphocytes in PP-HEVs. Micrographs of accumulated lymphocyte subpopulations in PPs. (Left panel) T cells accumulated in SLC+ interfollicular segments of HEVs. (Middle panel) B cells in SLClow HEV segments associated with primary follicles. (Right panel) Segregation of sites of preferential T and B cell accumulation in relation to follicles. TRITC-labeled T (left) or B (middle and right panels) cells and CMFDA-labeled T cells (right panel) were observed in HEVs of normal animals as in the legend to Fig. 4. In the left and middle panels, after lymphocyte accumulation for ∼10 min (see Materials and Methods), 60 μg Alexa™ 488–conjugated anti-SLC mAb was injected intravenously to illuminate SLC-expressing HEV segments. Animals were killed, and whole PPs were removed for examination by confocal microscopy. The location of follicles is indicated (F) (20× objective).
Similar articles
- The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules.
Stein JV, Rot A, Luo Y, Narasimhaswamy M, Nakano H, Gunn MD, Matsuzawa A, Quackenbush EJ, Dorf ME, von Andrian UH. Stein JV, et al. J Exp Med. 2000 Jan 3;191(1):61-76. doi: 10.1084/jem.191.1.61. J Exp Med. 2000. PMID: 10620605 Free PMC article. - Chemokine requirements for B cell entry to lymph nodes and Peyer's patches.
Okada T, Ngo VN, Ekland EH, Förster R, Lipp M, Littman DR, Cyster JG. Okada T, et al. J Exp Med. 2002 Jul 1;196(1):65-75. doi: 10.1084/jem.20020201. J Exp Med. 2002. PMID: 12093871 Free PMC article. - 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7.
Campbell JJ, Bowman EP, Murphy K, Youngman KR, Siani MA, Thompson DA, Wu L, Zlotnik A, Butcher EC. Campbell JJ, et al. J Cell Biol. 1998 May 18;141(4):1053-9. doi: 10.1083/jcb.141.4.1053. J Cell Biol. 1998. PMID: 9585422 Free PMC article. - Microanatomy of lymphocyte-endothelial interactions at the high endothelial venules of lymph nodes.
Tohya K, Umemoto E, Miyasaka M. Tohya K, et al. Histol Histopathol. 2010 Jun;25(6):781-94. doi: 10.14670/HH-25.781. Histol Histopathol. 2010. PMID: 20376785 Review. - Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues.
Ebert LM, Schaerli P, Moser B. Ebert LM, et al. Mol Immunol. 2005 May;42(7):799-809. doi: 10.1016/j.molimm.2004.06.040. Epub 2004 Nov 23. Mol Immunol. 2005. PMID: 15829268 Review.
Cited by
- Immune Responses to IAV Infection and the Roles of L-Selectin and ADAM17 in Lymphocyte Homing.
Reed SG, Ager A. Reed SG, et al. Pathogens. 2022 Jan 25;11(2):150. doi: 10.3390/pathogens11020150. Pathogens. 2022. PMID: 35215094 Free PMC article. Review. - Immune chemokines and their receptors: the key elements in the genesis, homeostasis and function of the immune system.
Yoshie O. Yoshie O. Springer Semin Immunopathol. 2000;22(4):371-91. doi: 10.1007/s002810000051. Springer Semin Immunopathol. 2000. PMID: 11155442 Review. No abstract available. - CCR7 in Blood Cancers - Review of Its Pathophysiological Roles and the Potential as a Therapeutic Target.
Cuesta-Mateos C, Terrón F, Herling M. Cuesta-Mateos C, et al. Front Oncol. 2021 Oct 29;11:736758. doi: 10.3389/fonc.2021.736758. eCollection 2021. Front Oncol. 2021. PMID: 34778050 Free PMC article. Review. - Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse.
Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Luther SA, et al. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12694-9. doi: 10.1073/pnas.97.23.12694. Proc Natl Acad Sci U S A. 2000. PMID: 11070085 Free PMC article.
References
- Bargatze R.F., Jutila M.A., Butcher E.C. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situthe multistep model confirmed and refined. Immunity. 1995;3:99–108 . - PubMed
- Butcher E.C., Williams M., Youngman K., Rott L., Briskin M. Lymphocyte trafficking and regional immunity. Adv. Immunol. 1999;72:209–253 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI37832/AI/NIAID NIH HHS/United States
- T32 AI007290/AI/NIAID NIH HHS/United States
- R37 GM037734/GM/NIGMS NIH HHS/United States
- GM37734/GM/NIGMS NIH HHS/United States
- 5532 AI07290/AI/NIAID NIH HHS/United States
- R01 GM037734/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous