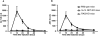Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis - PubMed (original) (raw)
Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis
Y Kaneko et al. J Exp Med. 2000.
Abstract
The administration of concanavalin A (Con A) induces a rapid severe injury of hepatocytes in mice. Although the Con A-induced hepatitis is considered to be an experimental model of human autoimmune hepatitis, the precise cellular and molecular mechanisms that induce hepatocyte injury remain unclear. Here, we demonstrate that Valpha14 NKT cells are required and sufficient for induction of this hepatitis. Moreover, interleukin (IL)-4 produced by Con A-activated Valpha14 NKT cells is found to play a crucial role in disease development by augmenting the cytotoxic activity of Valpha14 NKT cells in an autocrine fashion. Indeed, short-term treatment with IL-4 induces an increase in the expression of granzyme B and Fas ligand (L) in Valpha14 NKT cells. Moreover, Valpha14 NKT cells from either perforin knock-out mice or FasL-mutant gld/gld mice fail to induce hepatitis, and hence perforin-granzyme B and FasL appear to be effector molecules in Con A-induced Valpha14 NKT cell-mediated hepatocyte injury.
Figures
Figure 1
Elevation of serum transaminase activities after intravenous injection of Con A. Serum transaminase levels in B6 wild-type (•), Vα14 NKT KO (□), and RAG KO (▵) mice were assessed at different times after Con A (0.5 mg) administration. Three mice were used in each group. The mean transaminase activities (Karmen units per liter [K.U./l]) of ALT (A) and AST (B) of triplicate samples with standard errors are depicted.
Figure 2
Restoration of Con A–induced hepatitis by Vα14 NKT cells in Vα14 NKT KO mice. (A) TCR-β/NK1.1 profiles of liver mononuclear cells of Vα14 NKT KO mice 9 h after adoptive transfer of 2 × 107 spleen cells from Vα14 NKT mice are shown. The percentages of cells present in each area are shown in each panel. (B) The rearranged Vα14Jα281 gene was assessed by genomic PCR with specific primers detecting Vα14Jα281. Vα14 NKT KO mice were intrasplenically injected with the indicated numbers of freshly prepared whole spleen cells from Vα14 NKT mice (C) or Vα14 NKT cell line established from spleen cells (spl.) of Vα14 NKT mice (D). 1 h after cell transfer, the mice were administered Con A (0.5 mg; Stim.) intravenously. The serum ALT and AST activities were measured 8 h after Con A injection. Three mice were used in each group, and the serum samples were analyzed individually. The mean values of triplicate samples with standard errors are shown.
Figure 2
Restoration of Con A–induced hepatitis by Vα14 NKT cells in Vα14 NKT KO mice. (A) TCR-β/NK1.1 profiles of liver mononuclear cells of Vα14 NKT KO mice 9 h after adoptive transfer of 2 × 107 spleen cells from Vα14 NKT mice are shown. The percentages of cells present in each area are shown in each panel. (B) The rearranged Vα14Jα281 gene was assessed by genomic PCR with specific primers detecting Vα14Jα281. Vα14 NKT KO mice were intrasplenically injected with the indicated numbers of freshly prepared whole spleen cells from Vα14 NKT mice (C) or Vα14 NKT cell line established from spleen cells (spl.) of Vα14 NKT mice (D). 1 h after cell transfer, the mice were administered Con A (0.5 mg; Stim.) intravenously. The serum ALT and AST activities were measured 8 h after Con A injection. Three mice were used in each group, and the serum samples were analyzed individually. The mean values of triplicate samples with standard errors are shown.
Figure 2
Restoration of Con A–induced hepatitis by Vα14 NKT cells in Vα14 NKT KO mice. (A) TCR-β/NK1.1 profiles of liver mononuclear cells of Vα14 NKT KO mice 9 h after adoptive transfer of 2 × 107 spleen cells from Vα14 NKT mice are shown. The percentages of cells present in each area are shown in each panel. (B) The rearranged Vα14Jα281 gene was assessed by genomic PCR with specific primers detecting Vα14Jα281. Vα14 NKT KO mice were intrasplenically injected with the indicated numbers of freshly prepared whole spleen cells from Vα14 NKT mice (C) or Vα14 NKT cell line established from spleen cells (spl.) of Vα14 NKT mice (D). 1 h after cell transfer, the mice were administered Con A (0.5 mg; Stim.) intravenously. The serum ALT and AST activities were measured 8 h after Con A injection. Three mice were used in each group, and the serum samples were analyzed individually. The mean values of triplicate samples with standard errors are shown.
Figure 3
Development of Con A–induced hepatitis in Vα14 NKT mice. (A) Serum transaminase levels in B6 and Vα14 NKT mice 12 h after Con A administration (0.75 mg). All mice died within 24 h (†). The mean values of three samples with standard errors are shown. (B) Light micrographs of the liver with hematoxylin and eosin staining (×200 and ×400) are shown. The indicated mice were treated with 0.75 mg of Con A and killed 8 h later. Arrows indicate massive necrosis observed in the liver; arrowheads indicate mononuclear cell infiltration. WT, wild-type.
Figure 3
Development of Con A–induced hepatitis in Vα14 NKT mice. (A) Serum transaminase levels in B6 and Vα14 NKT mice 12 h after Con A administration (0.75 mg). All mice died within 24 h (†). The mean values of three samples with standard errors are shown. (B) Light micrographs of the liver with hematoxylin and eosin staining (×200 and ×400) are shown. The indicated mice were treated with 0.75 mg of Con A and killed 8 h later. Arrows indicate massive necrosis observed in the liver; arrowheads indicate mononuclear cell infiltration. WT, wild-type.
Figure 4
Production of IL-4 and IFN-γ from Con A–activated Vα14 NKT cells. Whole spleen cells from B6, Vα14 NKT KO, Vα14 NKT, and RAG KO mice (A) or the Vα14 NKT cell line and conventional T cell line (B) were stimulated in vitro with Con A (10 μg/ml). 24 h later, the amounts of IL-4 or IFN-γ in the culture supernatants were measured by ELISA. Mean values in triplicate samples with standard deviations are shown. n.d., not detectable.
Figure 5
Requirement of IL-4 produced by Vα14 NKT cells for Con A–induced hepatitis. (A) MACS-sorted NK1.1+ spleen cells (107) from B6, IL-4 KO, or IFN-γ KO mice were transferred intrasplenically into Vα14 NKT KO mice. 1 h later, the mice were injected with Con A (0.5 mg). The serum activities of ALT and AST were assessed 8 h after Con A injection. (B) Induction of Con A–induced hepatitis by adoptive transfer of cells from the Vα14 NKT cell line (2 × 106). Three mice were used in each group. The mean values in triplicate with standard errors are shown.
Figure 6
Augmentation of the cytotoxic activity of Vα14 NKT cells by short-term incubation with IL-4 in vitro. (A) Flow cytometric analysis of the expression of IL-4 and IFN-γRs on freshly isolated Vα14 NKT cells. (B) Spleen cells from Vα14 NKT mice were cultured for 8 h in the presence of 100 U/ml of IL-4 or 100 U/ml of IFN-γ. The cells were washed extensively and subjected to the standard 51Cr-release cytotoxic assay in triplicate on target TLR2 liver cells. (C) Augmented expression of mRNAs of granzyme B and FasL in Vα14 NKT cells upon stimulation with IL-4.
Figure 7
Requirement of FasL expression and perforin production from Vα14 NKT cells for Con A–induced hepatitis. MACS-sorted NK1.1+ spleen cells (107) from B6, B6-gld/gld, or perforin KO mice were transferred intrasplenically into Vα14 NKT KO mice. 1 h later, the mice were injected with Con A (0.5 mg). 8 h later, the serum activities of ALT and AST were assessed. The mean values of triplicate samples with standard errors are shown.
Similar articles
- Adhesion mediated by LFA-1 is required for efficient IL-12-induced NK and NKT cell cytotoxicity.
Matsumoto G, Omi Y, Lee U, Nishimura T, Shindo J, Penninger JM. Matsumoto G, et al. Eur J Immunol. 2000 Dec;30(12):3723-31. doi: 10.1002/1521-4141(200012)30:12<3723::AID-IMMU3723>3.0.CO;2-9. Eur J Immunol. 2000. PMID: 11169416 - IL-6 prevents T cell-mediated hepatitis via inhibition of NKT cells in CD4+ T cell- and STAT3-dependent manners.
Sun R, Tian Z, Kulkarni S, Gao B. Sun R, et al. J Immunol. 2004 May 1;172(9):5648-55. doi: 10.4049/jimmunol.172.9.5648. J Immunol. 2004. PMID: 15100309 - Essential role of the adhesion receptor LFA-1 for T cell-dependent fulminant hepatitis.
Matsumoto G, Tsunematsu S, Tsukinoki K, Ohmi Y, Iwamiya M, Oliveira-dos-Santos A, Tone D, Shindo J, Penninger JM. Matsumoto G, et al. J Immunol. 2002 Dec 15;169(12):7087-96. doi: 10.4049/jimmunol.169.12.7087. J Immunol. 2002. PMID: 12471145 - NK1.1+ T cell receptor-alpha/beta+ cells: new clues to their origin, specificity, and function.
MacDonald HR. MacDonald HR. J Exp Med. 1995 Sep 1;182(3):633-8. doi: 10.1084/jem.182.3.633. J Exp Med. 1995. PMID: 7650474 Free PMC article. Review. No abstract available. - Natural Killer T (NKT) Cells in Autoimmune Hepatitis: Current Evidence from Basic and Clinical Research.
Poddighe D, Maulenkul T, Zhubanova G, Akhmaldtinova L, Dossybayeva K. Poddighe D, et al. Cells. 2023 Dec 18;12(24):2854. doi: 10.3390/cells12242854. Cells. 2023. PMID: 38132174 Free PMC article. Review.
Cited by
- Intervention of PKC-θ as an immunosuppressive regimen.
Sun Z. Sun Z. Front Immunol. 2012 Aug 2;3:225. doi: 10.3389/fimmu.2012.00225. eCollection 2012. Front Immunol. 2012. PMID: 22876242 Free PMC article. - New Insight into the Concanavalin A-Induced Apoptosis in Hepatocyte of an Animal Model: Possible Involvement of Caspase-Independent Pathway.
Zhao X, Fu C, Sun L, Feng H, Xie P, Wu M, Tan X, Chen G. Zhao X, et al. Molecules. 2023 Jan 30;28(3):1312. doi: 10.3390/molecules28031312. Molecules. 2023. PMID: 36770978 Free PMC article. - A monoclonal antibody to the alpha2 domain of murine major histocompatibility complex class I that specifically kills activated lymphocytes and blocks liver damage in the concanavalin A hepatitis model.
Matsuoka S, Tsurui H, Abe M, Terashima K, Nakamura K, Hamano Y, Ohtsuji M, Honma N, Serizawa I, Ishii Y, Takiguchi M, Hirose S, Shirai T. Matsuoka S, et al. J Exp Med. 2003 Aug 4;198(3):497-503. doi: 10.1084/jem.20021301. Epub 2003 Jul 28. J Exp Med. 2003. PMID: 12885869 Free PMC article. - Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation.
Zheng X, Zhang H, Yin L, Wang CR, Liu Y, Zheng P. Zheng X, et al. PLoS One. 2008 Jul 16;3(7):e2703. doi: 10.1371/journal.pone.0002703. PLoS One. 2008. PMID: 18628995 Free PMC article. - Critical Contribution of NK Group 2 Member D Expressed on Invariant Natural Killer T Cells in Concanavalin A-Induced Liver Hepatitis in Mice.
Al Dulaimi D, Klibi J, Olivo Pimentel V, Parietti V, Allez M, Toubert A, Benlagha K. Al Dulaimi D, et al. Front Immunol. 2018 May 17;9:1052. doi: 10.3389/fimmu.2018.01052. eCollection 2018. Front Immunol. 2018. PMID: 29868013 Free PMC article.
References
- Makino Y., Kanno R., Ito T., Higashino K., Taniguchi M. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int. Immunol. 1995;7:1157–1161 . - PubMed
- Fowlkes B.J., Kruisbeek A.M., Ton-That H., Weston M.A., Coligan J.E., Schwartz R.H., Pardoll D.M. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly expresses a single Vβ gene family. Nature. 1987;329:251–254 . - PubMed
- Sykes M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+CD4−CD8− and αβTCR+NK1.1+ cells. J. Immunol. 1990;145:3209–3215 . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous