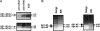Intraneuronal Abeta42 accumulation in human brain - PubMed (original) (raw)
doi: 10.1016/s0002-9440(10)64700-1.
J Tsai, J Naslund, B Vincent, M Edgar, F Checler, J P Greenfield, V Haroutunian, J D Buxbaum, H Xu, P Greengard, N R Relkin
Affiliations
- PMID: 10623648
- PMCID: PMC1868613
- DOI: 10.1016/s0002-9440(10)64700-1
Intraneuronal Abeta42 accumulation in human brain
G K Gouras et al. Am J Pathol. 2000 Jan.
Abstract
Alzheimer's disease (AD) is characterized by the deposition of senile plaques (SPs) and neurofibrillary tangles (NFTs) in vulnerable brain regions. SPs are composed of aggregated beta-amyloid (Abeta) 40/42(43) peptides. Evidence implicates a central role for Abeta in the pathophysiology of AD. Mutations in betaAPP and presenilin 1 (PS1) lead to elevated secretion of Abeta, especially the more amyloidogenic Abeta42. Immunohistochemical studies have also emphasized the importance of Abeta42 in initiating plaque pathology. Cell biological studies have demonstrated that Abeta is generated intracellularly. Recently, endogenous Abeta42 staining was demonstrated within cultured neurons by confocal immunofluorescence microscopy and within neurons of PS1 mutant transgenic mice. A central question about the role of Abeta in disease concerns whether extracellular Abeta deposition or intracellular Abeta accumulation initiates the disease process. Here we report that human neurons in AD-vulnerable brain regions specifically accumulate gamma-cleaved Abeta42 and suggest that this intraneuronal Abeta42 immunoreactivity appears to precede both NFT and Abeta plaque deposition. This study suggests that intracellular Abeta42 accumulation is an early event in neuronal dysfunction and that preventing intraneuronal Abeta42 aggregation may be an important therapeutic direction for the treatment of AD.
Figures
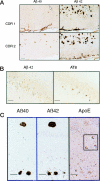
Figure 1.
Intraneuronal Aβ42 accumulation occurs in AD-vulnerable neurons before the formation of senile plaques. A, Left: Neuronal Aβ42 staining (RU antibody) in the CA1 region of hippocampus derived from a 64-year-old patient with mild (CDR 0.5) cognitive dysfunction. Right: Aβ40 staining from the same CA1 region shows only slight immunoreactivity compared with the more pronounced intracytoplasmic staining seen with Aβ42. Antibody concentrations and time of development were equivalent. Bar = 60 μm. B, Left: Aβ42 immunoreactivity (RU antibody) in basal forebrain magnocellular neurons. Right: This staining is abolished by Aβ1–42 peptide competition; a blue filter was used to highlight negatively staining neurons. Bar = 60 μm. C, Left: Aβ42 staining (QCB) in the CA4 region of hippocampus from a neurologically normal 3-year-old patient (control); only faint neuronal staining can be seen (left), Bar = 60 μm. Center: Pronounced CA4 Aβ42 immunoreactivity (QCB) in a 3-year-old with Down’s syndrome. The arrow indicates a neuron with intracellular staining. Bar = 40 μm. Right: Aβ42 staining (QCB) in a 79-year-old without dementia indicates marked Aβ42 intracellular staining in layer II neurons (arrows) of the entorhinal cortex. Bar = 100 μm. D, Left: In this 83-year-old cognitively impaired subject (CDR1), the absence of intranuclear Aβ42 staining is evident in neurons stained for Aβ42 (RU). Early Aβ42 aggregates appear to be present within a neuron marked by an arrow; the inset provides another example of such seemingly intracellular Aβ42 accumulation in (RU) in a 94-year-old CDR 2 case. Bar = 40 μm. Center: “Neuronal” shaped SP (arrow) adjacent to a more conventional spherical SP in a 72-year-old subject with advanced AD (RU Aβ42). Bar = 60 μm. Right: The CA1 region of a 79-year-old cognitively impaired subject (CDR1) demonstrates both intraneuronal Aβ42 immunoreactivity (QCB) and apparent extraneuronal diffuse plaque-like staining (arrow) adjacent to a few neurons. Bar = 40 μm.
Figure 2.
A: Intraneuronal Aβ42 immunoreactivity (QCB) in layer II (islands of Calleja) of the entorhinal cortex (arrow) in a 90-year-old CDR1 patient, compared with the absence of staining (arrow) in an 83-year-old CDR2 patient; Aβ42 immunoreactive plaques can be seen above. In the CDR 2 patient, note the emergence of Aβ40 SPs. Bar = 100 μm. B: Abundant Aβ42 immunoreactivity (RU) compared with only occasional AT8 staining for hyperphosphorylated tau in the CA1 region of a 94-year-old patient (CDR 2). Bar = 60 μm. C: Adjacent sections of CA4 (below) and dentate gyrus (above) immunostained with antibodies to Aβ40, Aβ42 (QCB), and apoE in an 83-year-old cognitively impaired patient (CDR 2). Noticeable intraneuronal apoE staining is evident (inset, enlarged ×5). Bar = 100 μm.
Figure 3.
Metabolic labeling and immunoprecipitation of intraneuronal Aβ40 and Aβ42. A: Primary mouse neuronal cultures. Top: IP of conditioned medium indicates significantly lower secretion of Aβ42 compared with Aβ40. Bottom: Comparable amounts of Aβ40 and Aβ42 species in neuronal cell lysate. B: Aβ40 and Aβ42 species in sucrose density gradients from neuroblastoma cells harboring the Δ10eFAD PS1 mutation. Aβ1–40, Aβx-40, and Aβ1–42 species predominate in the Golgi-enriched fraction, whereas Aβx-42 predominates in the ER-enriched fraction.
Similar articles
- Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer's disease.
Takahashi RH, Nagao T, Gouras GK. Takahashi RH, et al. Pathol Int. 2017 Apr;67(4):185-193. doi: 10.1111/pin.12520. Epub 2017 Mar 5. Pathol Int. 2017. PMID: 28261941 Review. - Intraneuronal Abeta42 accumulation in Down syndrome brain.
Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ, Lemere CA. Mori C, et al. Amyloid. 2002 Jun;9(2):88-102. Amyloid. 2002. PMID: 12440481 - Significance of intracellular Abeta42 accumulation in Alzheimer's disease.
Tabira T, Chui DH, Kuroda S. Tabira T, et al. Front Biosci. 2002 Apr 1;7:a44-9. doi: 10.2741/tabira. Front Biosci. 2002. PMID: 11897569 - Intraneuronal Aβ accumulation, amyloid plaques, and synapse pathology in Alzheimer's disease.
Capetillo-Zarate E, Gracia L, Tampellini D, Gouras GK. Capetillo-Zarate E, et al. Neurodegener Dis. 2012;10(1-4):56-9. doi: 10.1159/000334762. Epub 2012 Jan 21. Neurodegener Dis. 2012. PMID: 22269167 - Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer's disease pyramidal neurons.
D'Andrea MR, Nagele RG. D'Andrea MR, et al. Curr Pharm Des. 2006;12(6):677-84. doi: 10.2174/138161206775474224. Curr Pharm Des. 2006. PMID: 16472157 Review.
Cited by
- Overview of Alzheimer's Disease and Some Therapeutic Approaches Targeting Aβ by Using Several Synthetic and Herbal Compounds.
Singh SK, Srivastav S, Yadav AK, Srikrishna S, Perry G. Singh SK, et al. Oxid Med Cell Longev. 2016;2016:7361613. doi: 10.1155/2016/7361613. Epub 2015 Dec 28. Oxid Med Cell Longev. 2016. PMID: 27034741 Free PMC article. Review. - Autophagy Markers Are Altered in Alzheimer's Disease, Dementia with Lewy Bodies and Frontotemporal Dementia.
Longobardi A, Catania M, Geviti A, Salvi E, Vecchi ER, Bellini S, Saraceno C, Nicsanu R, Squitti R, Binetti G, Di Fede G, Ghidoni R. Longobardi A, et al. Int J Mol Sci. 2024 Jan 17;25(2):1125. doi: 10.3390/ijms25021125. Int J Mol Sci. 2024. PMID: 38256197 Free PMC article. - Mechanisms of Pathogenic Tau and Aβ Protein Spreading in Alzheimer's Disease.
d'Errico P, Meyer-Luehmann M. d'Errico P, et al. Front Aging Neurosci. 2020 Aug 27;12:265. doi: 10.3389/fnagi.2020.00265. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33061903 Free PMC article. Review. - A Chronic Increase in Blood-Brain Barrier Permeability Facilitates Intraneuronal Deposition of Exogenous Bloodborne Amyloid-Beta1-42 Peptide in the Brain and Leads to Alzheimer's Disease-Relevant Cognitive Changes in a Mouse Model.
Acharya NK, Grossman HC, Clifford PM, Levin EC, Light KR, Choi H, Swanson Ii RL, Kosciuk MC, Venkataraman V, Libon DJ, Matzel LD, Nagele RG. Acharya NK, et al. J Alzheimers Dis. 2024;98(1):163-186. doi: 10.3233/JAD-231028. J Alzheimers Dis. 2024. PMID: 38393907 Free PMC article. - A Combination of Heavy Metals and Intracellular Pathway Modulators Induces Alzheimer Disease-like Pathologies in Organotypic Brain Slices.
Korde DS, Humpel C. Korde DS, et al. Biomolecules. 2024 Jan 30;14(2):165. doi: 10.3390/biom14020165. Biomolecules. 2024. PMID: 38397402 Free PMC article.
References
- Selkoe DJ: The cell biology of β-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol 1998, 8:447-453 - PubMed
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y: Visualization of A β 42(43) and A β 40 in senile plaques with end-specific A β monoclonals: evidence that an initially deposited species is A β 42(43). Neuron 1994, 13:45-53 - PubMed
- Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ: Sequence of deposition of heterogeneous amyloid β-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis 1996, 3:16-32 - PubMed
- Wild-Bode C, Yamazaki T, Capell A, Leimer U, Steiner H, Ihara Y, Haass C: Intracellular generation and accumulation of amyloid β-peptide terminating at amino acid 42. J Biol Chem 1997, 272:16085-16088 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS02037/NS/NINDS NIH HHS/United States
- AG05138/AG/NIA NIH HHS/United States
- P01 AG009464/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- AG09464/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources