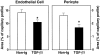Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice - PubMed (original) (raw)
Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice
T Wyss-Coray et al. Am J Pathol. 2000 Jan.
Abstract
Cerebrovascular amyloid deposition and microvascular degeneration are frequently associated with Alzheimer's disease (AD), but the etiology and pathogenetic role of these abnormalities are unknown. Recently, transforming growth factor-beta1 (TGF-beta1) was implicated in cerebrovascular amyloid formation in transgenic mice with astroglial overproduction of TGF-beta1 and in AD. We tested whether TGF-beta1 overproduction induces AD-like cerebrovascular degeneration and analyzed how cerebrovascular abnormalities develop over time in TGF-beta1-transgenic mice. In cerebral microvessels from 3- to 4-month-old TGF-beta1-transgenic mice, which display a prominent perivascular astrocytosis, levels of the basement membrane proteins perlecan and fibronectin were severalfold higher than in vessels from nontransgenic mice. Consistent with this increase, cortical capillary basement membranes of TGF-beta1 mice were significantly thickened. These changes preceded amyloid deposition, which began at around 6 months of age. In 9- and 18-month-old TGF-beta1 mice, various degenerative changes in microvascular cells of the brain were observed. Endothelial cells were thinner and displayed abnormal, microvilli-like protrusions as well as occasional condensation of chromatin, and pericytes occupied smaller areas in capillary profiles than in nontransgenic controls. Similar cerebrovascular abnormalities have been reported in AD. We conclude that chronic overproduction of TGF-beta1 triggers an accumulation of basement membrane proteins and results in AD-like cerebrovascular amyloidosis and microvascular degeneration. Closely related processes may induce cerebrovascular pathology in AD.
Figures
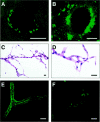
Figure 1.
Thioflavin S-positive deposits in cerebral blood vessels of TGF-β1 mice and AD. Cortical brain sections from a 12-month-old TGF-β1 (line T115 heterozygous) mouse (A) and an AD patient with a CAA score of 3 (B) were stained with thioflavin S and analyzed by confocal microscopy. Thioflavin S-positive amyloid deposits are located on the abluminal side of endothelial cells in TGF-β1 mice and the AD case. Isolated cerebral blood vessels stained with H&E from an 18-month-old TGF-β1 mouse were examined at low-power (C) or high-power (D) magnification. No gross structural abnormalities were observed. E and F: Thioflavin S-stained isolated cerebral blood vessels from an 18-month-old TGF-β1 mouse with various degrees of amyloid deposition. Scale bars, 20 μm.
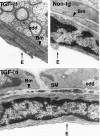
Figure 2.
Electron-dense deposits within the basement membrane of cerebral blood vessels in TGF-β1 mice. Ultrathin brain sections from a 9-month-old TGF-β1 mouse (line T64 heterozygous) and a nontransgenic littermate control (Non-tg) were analyzed by electron microscopy. Basement membrane (Bm) of a medium-sized blood vessel from a TGF-β1 mouse shows electron-dense deposits (edd) at medium- and high-power magnification. No such deposits were observed in nontransgenic blood vessels. E, endothelial cell; SM, smooth muscle cell. (Original magnifications: top left, ×31,500; top right and bottom, ×42,500).
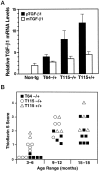
Figure 3.
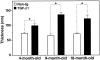
Accumulation of thioflavin S-positive deposits in TGF-β1 mice depends on age and level of TGF-β1 expression. Brains from TGF-β1 line T64 heterozygous mice (T64−/+), line T115 heterozygous mice (T115−/+), and line T115 homozygous mice (T115+/+) and nontransgenic littermate controls (Non-tg) were analyzed for cerebral TGF-β1 mRNA expression levels and for the number of thioflavin S-positive cerebrovascular deposits. A: Total RNA was extracted 3 months postnatally (n = 4 mice per group). The relative levels of porcine (transgene) TGF-β1 (pTGF-β1) and murine (endogenous) TGF-β1 (mTGF-β1) were determined by RNase protection assay. Results are means ± SEMs obtained by phosphorimager analysis and normalization to actin values. B: Sagittal brain sections from the indicated groups of mice (aged 3–6, 9–12, or 15–18 months) were stained with thioflavin S and examined by fluorescence microscopy. Data points represent mean scores from three to six sections per mouse. Older mice had more thioflavin S-positive deposits than younger mice with similar levels of transgene expression, and higher levels of TGF-β1 expression resulted in more amyloid deposits.
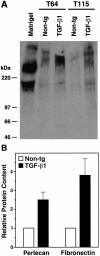
Figure 4.
Increased perlecan and fibronectin levels in cerebral microvessels from young TGF-β1 mice. A: Western blot analysis showing perlecan expression in homogenates from isolated cortical and hippocampal microvessels (180 μg per lane) from 4-month-old TGF-β1 mice (line T64 or T115 heterozygous) or nontransgenic littermate controls. A mix of extracellular matrix proteins (Matrigel, 1 μl) served as a positive control. Microvessels prepared from TGF-β1 transgenic or nontransgenic control brains (n = 5 per group) were pooled yielding approximately 300 μg of protein per group. Molecular size standards shown on the left indicate perlecan-immunoreactive products in cerebral microvessels exeeding 400 kd, as well as a smaller-size protein of approximately 200 kd, which is also present in Matrigel. B: Perlecan and fibronectin contents determined semiquantitatively by densitometric analysis in three to four independent experiments were significantly higher in TGF-β1–transgenic (line T64) than in nontransgenic microvessels (*P < 0.05; Mann-Whitney U test). Results are means ± SEMs.
Figure 5.
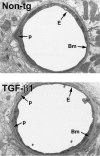
Ultrastructural abnormalities in cerebral blood vessels of TGF-β1 mice. Ultrathin brain sections from 9-month-old TGF-β1 mice (line T64 heterozygous) or nontransgenic littermate controls (Non-tg) were analyzed by electron microscopy. In the TGF-β1 transgenic vessel, the basement membrane (Bm) appears thickened, and the endothelial cell (E) profile is thinner with an irregular luminal surface. The nontransgenic control vessel has a normal basement membrane and a smooth endothelial cell surface. Original magnifications, ×5000. p, pericyte.
Figure 6.
Age-related accumulation of basement membrane proteins in cortical capillaries of TGF-β1 mice. Ultrathin cortical brain sections from TGF-β1 mice (line T64 heterozygous) and nontransgenic littermate controls (Non-tg) at 4, 9, and 18 months of age were analyzed by electron microscopy. The thickness of the basement membrane was measured in seven to nine capillary profiles per brain in a total of three mice per group by computer-assisted morphometry as described in Materials and Methods. Results are means ± SEMs. Basement membranes were significantly thicker in capillaries from TGF-β1 brains than in capillaries from nontransgenic control brains (*P < 0.005; unpaired, two-tailed Student’s _t_-test).
Figure 7.
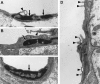
Microvascular endothelial cell damage in brains of 9-month-old TGF-β1 mice. Ultrathin cortical brain sections from TGF-β1 mice (line T64 heterozygous; A, B, D) and nontransgenic littermate controls (C) were analyzed by electron microscopy. Endothelial cell nuclei (arrows) from TGF-β1 brains showed signs of increased chromatin condensation (A, B), whereas the nucleus from a nontransgenic control appeared normal (C). Frequently, the surface of TGF-β1 transgenic endothelial cells was irregular (A, B, D), with microvilli-like protrusions and bleb-like structures (arrowheads). Original magnifications, ×31,500 (A−C) and ×72,000 (D).
Figure 8.
Abnormalities in brain capillaries from TGF-β1 mice. The relative areas occupied by endothelial cell profiles (left) or pericyte profiles (right) were measured in cross-sections of cortical capillaries from 9-month-old TGF-β1 mice (line T64 heterozygous) and nontransgenic littermate controls. Results are means ± SDs from seven to nine capillaries analyzed in three mice per group. The relative areas of endothelial cell and pericyte profiles in 9-month-old TGF-β1 mice were significantly smaller than those in controls (*P < 0.05; unpaired, two-tailed Student’s _t_-test).
Similar articles
- Alzheimer's disease-like cerebrovascular pathology in transforming growth factor-beta 1 transgenic mice and functional metabolic correlates.
Wyss-Coray T, Lin C, von Euw D, Masliah E, Mucke L, Lacombe P. Wyss-Coray T, et al. Ann N Y Acad Sci. 2000 Apr;903:317-23. doi: 10.1111/j.1749-6632.2000.tb06382.x. Ann N Y Acad Sci. 2000. PMID: 10818521 - Molecular and functional dissection of TGF-beta1-induced cerebrovascular abnormalities in transgenic mice.
Buckwalter M, Pepper JP, Gaertner RF, Von Euw D, Lacombe P, Wyss-Coray T. Buckwalter M, et al. Ann N Y Acad Sci. 2002 Nov;977:87-95. doi: 10.1111/j.1749-6632.2002.tb04801.x. Ann N Y Acad Sci. 2002. PMID: 12480736 Review. - Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer's disease.
Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L. Wyss-Coray T, et al. Nature. 1997 Oct 9;389(6651):603-6. doi: 10.1038/39321. Nature. 1997. PMID: 9335500 - Cerebrovascular pathology during the progression of experimental Alzheimer's disease.
Giannoni P, Arango-Lievano M, Neves ID, Rousset MC, Baranger K, Rivera S, Jeanneteau F, Claeysen S, Marchi N. Giannoni P, et al. Neurobiol Dis. 2016 Apr;88:107-17. doi: 10.1016/j.nbd.2016.01.001. Epub 2016 Jan 8. Neurobiol Dis. 2016. PMID: 26774030 - TGF-β1 factor in the cerebrovascular diseases of Alzheimer's disease.
Zhang X, Huang WJ, Chen WW. Zhang X, et al. Eur Rev Med Pharmacol Sci. 2016 Dec;20(24):5178-5185. Eur Rev Med Pharmacol Sci. 2016. PMID: 28051272 Review.
Cited by
- Neuroinflammatory processes in Alzheimer's disease.
Heneka MT, O'Banion MK, Terwel D, Kummer MP. Heneka MT, et al. J Neural Transm (Vienna). 2010 Aug;117(8):919-47. doi: 10.1007/s00702-010-0438-z. Epub 2010 Jul 15. J Neural Transm (Vienna). 2010. PMID: 20632195 Review. - MRI Types of Cerebral Small Vessel Disease and Circulating Markers of Vascular Wall Damage.
Dobrynina LA, Zabitova MR, Shabalina AA, Kremneva EI, Akhmetzyanov BM, Gadzhieva ZS, Berdalin AB, Kalashnikova LA, Gnedovskaya EV, Krotenkova MV. Dobrynina LA, et al. Diagnostics (Basel). 2020 May 29;10(6):354. doi: 10.3390/diagnostics10060354. Diagnostics (Basel). 2020. PMID: 32485815 Free PMC article. - TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons.
Kandasamy M, Lehner B, Kraus S, Sander PR, Marschallinger J, Rivera FJ, Trümbach D, Ueberham U, Reitsamer HA, Strauss O, Bogdahn U, Couillard-Despres S, Aigner L. Kandasamy M, et al. J Cell Mol Med. 2014 Jul;18(7):1444-59. doi: 10.1111/jcmm.12298. Epub 2014 Apr 30. J Cell Mol Med. 2014. PMID: 24779367 Free PMC article. - The role of resveratrol as a natural modulator in glia activation in experimental models of stroke.
Ghazavi H, Shirzad S, Forouzanfar F, Sahab Negah S, Riyahi Rad M, Vafaee F. Ghazavi H, et al. Avicenna J Phytomed. 2020 Nov-Dec;10(6):557-573. Avicenna J Phytomed. 2020. PMID: 33299813 Free PMC article. Review. - Proportions of Basement Membrane Proteins in Cerebrovascular Smooth Muscle Cells After Exposure to Hypercapnia and Amyloid Beta.
Dewing JM, Keable A, Laslo A, Chinezu L, Ivanescu A, Ratnayaka JA, Kalaria R, Slevin M, Verma A, Carare RO. Dewing JM, et al. Cells. 2025 Apr 18;14(8):614. doi: 10.3390/cells14080614. Cells. 2025. PMID: 40277938 Free PMC article.
References
- Kalaria RN: Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther 1996, 72:193-214 - PubMed
- Vinters HV: Cerebral amyloid angiopathy and Alzheimer’s disease: two entities or one? J Neurol Sci 1992, 112:1-3 - PubMed
- Plassman BL, Breitner JCS: Recent advances in the genetics of Alzheimer’s disease and vascular dementia with an emphasis on gene-environment interactions. J Am Geriatr Soc 1996, 44:1242-1250 - PubMed
- Verbeek MM, Eikelenboom P, De Waal RMW: Differences between the pathogenesis of senile plaques and congophilic angiopathy in Alzheimer disease. J Neuropathol Exp Neurol 1997, 56:751-761 - PubMed
- Slooter AJC, Tang MX, Van Duijn CM, Stern Y, Ott A, Bell K, Breteler MMB, Van Broeckhoven C, Tatemichi TK, Tycko B, Hofman A, Mayeux R: Apolipoprotein E (ε4), and the risk of dementia with stroke: a population-based investigation. J Am Med Assoc 1997, 277:818-821 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG10689/AG/NIA NIH HHS/United States
- R01 AG011385/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- AG15871/AG/NIA NIH HHS/United States
- R37 AG011385/AG/NIA NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases