Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer - PubMed (original) (raw)
Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer
S Higashijima et al. J Neurosci. 2000.
Abstract
We generated germ line-transmitting transgenic zebrafish that express green fluorescent protein (GFP) in the cranial motor neurons. This was accomplished by fusing GFP sequences to Islet-1 promoter/enhancer sequences that were sufficient for neural-specific expression. The expression of GFP by the motor neurons in the transgenic fish enabled visualization of the cell bodies, main axons, and the peripheral branches within the muscles. GFP-labeled motor neurons could be followed at high resolution for at least up to day four, when most larval neural circuits become functional, and larvae begin to swim and capture prey. Using this line, we analyzed axonal outgrowth by the cranial motor neurons. Furthermore, by selective application of DiI to specific GFP-positive nerve branches, we showed that the two clusters of trigeminal motor neurons in rhombomeres 2 and 3 innervate different peripheral targets. This finding suggests that the trigeminal motor neurons in the two clusters adopt distinct fates. In future experiments, this transgenic line of zebrafish will allow for a genetic analysis of cranial motor neuron development.
Figures
Fig. 1.
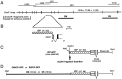
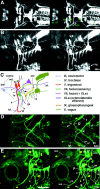
Genomic structure of the Islet-1(Isl1) gene and the plasmid constructs.A, _Eco_RI restriction map of the genomic DNA flanking the Isl1 gene. Lines over the map represent phage clones (LS1, LS2,and LS22) and a BAC clone (N21). The_thick line, CM, includes the enhancer elements for driving GFP expression in cranial motor neurons, whereas_SS includes the enhancer elements for driving GFP expression in the trigeminal ganglion cells and Rohon–Beard cells. Other Eco_RI fragments were negative for enhancer activity by our transient expression assay. B, Close-up view of the genomic region around the Isl1 promoter.Filled boxes are protein coding regions. The_hatched box represents the putative Isl1_promoter (ICP), which contains ∼4.1 kb of the 5′ upstream region and an ∼30 bp of the 5′ untranslated region of the_Isl1 gene. It was used in all the constructs as a core promoter. C, A map of the ICP–GFP, the core plasmid. Every genomic _Eco_RI fragment was inserted into the_Eco_RI site located in the immediate upstream of ICP._Not_I was used to linearize the plasmid DNA for microinjection. D, A map of the CMICP–GFP or SSICP–GFP (containing the SS-fragment) plasmid. The CMICP–GFP plasmid drove expression of GFP in the cranial motor neurons, and it was used for generating the Isl1-GFP line. The SSICP–GFP plasmid drove expression of GFP both in Rohon–Beard neurons and the trigeminal sensory neurons.
Fig. 2.
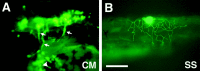
Zebrafish _Isl1_promoter/enhancer activity in transient expression assays.A, GFP expression in the head of a 40 hr embryo injected with the CMICP–GFP plasmid. The arrows indicate GFP expression in branchial motor nerves. The _arrowhead_indicates GFP expression in the hatching gland cells. Anterior is to the left, dorsal is up. _B,_A lateral view of GFP expression in a Rohon–Beard cell in the spinal cord of a 36 hr embryo injected with the SSICP–GFP plasmid. Scale bar:A, 200 μm; B, 230 μm.
Fig. 3.
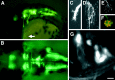
GFP expression in cranial motor neurons in the Isl1-GFP line. A, Lateral view of the head region of a 34 hr embryo. The arrow indicates GFP expression in the hatching gland cells. B, Dorsal view of the midbrain and the hindbrain of a 42 hr embryo. In A and_B_, pictures were taken under a conventional epifluorescence microscope. C–G, Close-up views of the GFP-positive nerve endings and neurons in the Isl1-GFP line using a confocal microscope. In panels C–F, composite pictures were generated from the stacked confocal images. C,Lateral view of the distal tips of the trigeminal motor axons at 30 hr. Note the fine filopodial structures extending from the distal ends of the axons. D, Lateral view of the neuromuscular junction in the levator arcus palatini (lap) muscle at 96 hr. Note the fine arborization of the nerve branches innervating individual muscle fibers. E, F, GFP expression in the efferent component of the lateral line nerves in a 96 hr embryo. Close-up view of their endings at the neuromasts. The position of the neuromast corresponds to_h in Figure 7_C. In F, the larva was treated with the vital dye DASPEI, which stains hair cells. DASPEI signals appear as orange. Note that the nerve endings appear to terminate within the hair cells. G, A confocal optical section showing the trigeminal motor neurons at 105 hr. Dorsal view with medial to the top. A cluster of neurons situated on the_right_ correspond to those in the posterior (Vp) cluster of the trigeminal motor neurons (Figs. 5-7). Note that individual GFP-positive neurons are readily visualized. In all figures, anterior is to the left. In lateral views, dorsal is_up_. Scale bar: A, 110 μm;B, 60 μm; C–F, 20 μm;G, 10 μm.
Fig. 4.
Comparison of the expression patterns of_Islet-1_ (Isl1) and _GFP_mRNAs in the cranial motor and sensory neurons. A,C, E, GFP mRNA expression in embryos from the Isl1-GFP line. B, D,F, Isl1 mRNA expression in wild-type embryos. A, B, Dorsal views of 28 hr embryos.GFP and Isl1 mRNAs are both expressed in presumptive cranial motor neurons. Whereas Isl1 mRNA is expressed in the trigeminal ganglion cells (tg),GFP mRNA is not expressed in these cells. C, D, Dorsal views of 40 hr embryos. Isl1 mRNA expression in the nIII and nIV neurons is difficult to see because many other neighboring cells also express Isl1 mRNA (D, asterisk). Arrows in _D_indicate cells that do not express GFP mRNA in the Isl1-GFP line. The nVI and nIX neurons do not express GFP in the Isl1-GFP zebrafish (see the following figures). _E, F,_Lateral views of 40 hr embryos. GFP is expressed in cells in the facial sensory ganglion (fs), glossopharyngeal sensory ganglion (gs), and vagus sensory ganglions (vs), but is not expressed in cells in the trigeminal sensory ganglion (tg). Scale bar, 100 μm.
Fig. 5.
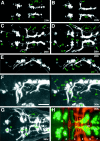
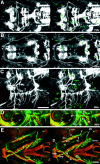
GFP expression early in the development of the cranial motor neurons (21–36 hr) in the Isl1-GFP line. All micrographs are confocal composite images generated from a series of optical sections. E and F are stereographic images. A, Dorsal view of a 21 hr embryo. The arrow indicates the nVII/OLe axons. At this stage most of the GFP-positive nVII/OLe neurons are located in r4 and r5. Rhombomere boundaries were estimated by designating the hindbrain region spanning the central half of the otocyst (oto) as r5, and those spanning the anterior and posterior quarters of the otocyst as parts of r4 and r6, respectively. B, Dorsal view of a 24 hr embryo. The pioneering axons from the nVII/OLe neurons are seen outside the hindbrain (arrow).C, Dorsal view of a 26 hr embryo. *Longitudinal processes that connect the nIII, nV, and nVII motor nuclei are visible (for example, a and b). *The significance of these early-forming axons is unknown. The majority of the nVII/OLe axons exit from the hindbrain (c).f indicates anteriorly projecting axons that subsequently extend into the lateral line system (corresponding to_l_ in E). D, Dorsal view of a 28 hr embryo. g indicates axons from the nIII neurons.h indicates the peripherally extending axons from the Va cluster of the nV neurons. d in C and_i_ in D indicate efferent axons extending into the posterior lateral line. *Efferent axons for the posterior lateral line also exit from the hindbrain via a posteriorly located exit point (e in C and j_in D; Metcalfe et al., 1985). *Arrowheads_in A–D indicate GFP-positive cells located anteriorly to the main nV cluster. *It is not clear whether these cells constitute a part of the nV neurons. E, Lateral view of a 28 hr embryo. k indicates the peripherally extending axons from the nV neurons. l indicates diverging axons extending into the lateral line system, whereas m_indicates the main nVII/OLe axons (mostly, the facial motor axons). It should be noted that, viewed laterally, the efferent axons for the posterior lateral line appear interrupted by the otocyst because of the opaque nature of the otolith (also in Figs. 5_F, 6_B,E, 7_C).F, Lateral view of a 36 hr embryo. n_indicates dorsally extending axons from the nIV neurons.o and p indicate the peripherally extending axons from the nV and nVII/OLe neurons, respectively.G, Dorsal view of a 36 hr embryo. The thick arrow indicates axons from the Vp cluster of the nV neurons. *Contralaterally projecting neurites from the nVII/OLe neurons are visible (asterisk). H, A confocal composite image of a 36 hr embryo in which hindbrain commissural axons that are located at the rhombomere boundary are labeled with the zn5 antibody. Dorsal view. Numbers listed are rhombomere numbers. Green is the GFP signal, whereas_red is the zn5 signal. VII and_VII′_, VII/OLe complex; oto, otocyst;Va, Vp, anterior and posterior clusters of nV neurons, respectively; fs, facial sensory ganglion;pL, posterior lateral line; soL, supraorbital lateral line; ioL, infraorbital lateral line; gs, glossopharyngeal sensory ganglion;hg, hatching gland. The sentences denoted by an_asterisk_ in this and all other legends describe observations not detailed in Results. In lateral views, dorsal is to the top. Scale bar, _A–G,_100 μm; H, 50 μm.
Fig. 6.
GFP expression in the Isl1-GFP line on days 2 and 3 of development. All panels except C are confocal stereographic images. A, Dorsal view of a 48 hr embryo. The asterisk indicates medially located nV neurons that are presumably born later than laterally located nV neurons. B, Lateral view of a 48 hr embryo.C, Schematic illustration of GFP-labeled nerves in_B_. ipsi and contra_indicate fourth nerves from the nIV neurons on the ipsilateral and the contralateral sides, respectively. a and_b indicate the peripherally projecting axons from the facial sensory ganglion cells. The axons of a take an internal pathway, whereas those of b take an external pathway. The axons indicated by a and b,respectively, correspond to those indicated by in and_ex in Figure 9_A–C. Thin arrows show GFP-expressing cells that presumably do not correspond to motor neurons. The thick arrow indicates an OLe nerve branch extending into the hair cells in the otocyst. The_asterisk_ indicates branching processes of the OLe nerves, which are added later to the major branches. The_arrowhead_ indicates the distal tip of the fifth motor axons. D, Ventral view of a 62 hr embryo.E, Lateral view of a 72 hr larva. The embryo in_D_ and the larva in E were treated with the vital dye DASPEI, which stains hair cells in lateral line neuromasts. DASPEI signals appear as yellow.I, D, and P indicate the intermediate, distal, and proximal branches of the fifth motor nerve, respectively. Arrows in D and_E_ indicate peripherally projecting axons from the facial sensory ganglion cells (equivalent to a and_b_ in C). Among them, in_indicates the axons that are distal tip of the axons indicated as_a in C. They take an internal pathway. Most part of their tract is out-of-focus. The arrowhead_indicates the junction of the four motor nerves (both sides of the fifth and seventh motor nerves). c and d_indicate nerve branches that innervate the intermandibularis anterior and posterior (ima and imp), and the hyohyal (hh), respectively (Fig. 7_E).e indicates the neuromuscular junction at the levator arcus palatini (lap), whereas f indicates the neuromuscular junctions at the abductor hyomandibulae (ah) and the abductor operculi (ao) (Fig.7_D). g indicates distal branches of OLe nerves from the main nVII/OLe nerve. Asterisks indicate nerve endings of the OLe system in the ear (Fig. 7_C_). *cn is likely to be the ciliary nerve innervating the lens muscle for visual accommodation. *The lens muscle in teleost was shown to be controlled by the postganglionic fibers of the oculomotor (parasympathetic) nerve (Somiya, 1987), and thus, GFP should be expressed in the parasympathetic ganglion cells corresponding to the ciliary nerve. a-fs, centrally projecting afferent facial sensory axons; pL, posterior lateral line;vs, vagus sensory ganglion. Scale bar, 100 μm.
Fig. 7.
GFP expression in the Isl1-GFP line on days 3 and 4 of development. All figures are stereographic pictures reconstructed from the stacked confocal images. A,Dorsal view of a 72 hr larva. B, Dorsal view of a 96 hr larva. GFP is also expressed in cells other than motor neurons in the brain. The identities of these cells are not known. They are a cluster of cells in the telencephalon (b) and a cluster of cells in the mesencephalic region (d). Tracts are formed between these two clusters (c). The cluster of the mesencephalic region (d) also sends processes posteriorly (e). a_in A corresponds to d in_B. For these cells and tracts, see also the legend to Figure 9_E. C, Lateral view of a 96 hr larva. Five terminal-like structures are observed in the ear. These appear to correspond to the anterior macula (ma) accompanied by the anterior otolith (the anteriorly located triangle), the medial macula (mm) accompanied by the posterior otolith (the posteriorly located triangle), and the three crista ampullarises (asterisks) associated with the anterior, lateral, and posterior canals. Nomenclatures are according to Whitfield et al. (1996). f indicates the largest vagus sensory ganglion, which is likely to include neurons innervating visceral organs. g indicates the vagus nerve extending into the visceral organs. The nerve is likely to contain sensory components and visceral motor (parasympathetic) components. h indicates the endings of the OLe nerve at a lateral line neuromast. Higher magnification views of the corresponding structures are shown in Figure3, E and F. In B and_C, elaborated fine branches are visible at neuromuscular junctions (for example, so and sr in_B_, lap in C). D, E, Fixed larvae were treated with rhodamine–phalloidin to reveal actin filaments of muscles. Green is the GFP signal, whereas red is the rhodamine–phalloidin signal.D, Lateral view of a 96 hr larva. E,Ventral view of a 105 hr larva. P, I, and_D in C–E indicate the proximal, intermediate, and distal branches of the fifth motor nerve, respectively. The proximal branch of the fifth nerve innervates the_lap and do muscles, the intermediate branch innervates the am muscle, and the distal branch innervates the ima and imp muscles. The configuration is schematically summarized in Figure 8_A. Arrows_ indicate peripherally projecting axons from the facial sensory ganglion cells. *The seventh motor nerves innervate surfacial membranous muscles (indicated as m-m), which presumably corresponds to the platysma in higher vertebrates. The_arrowhead_ indicates the junction of the four motor nerves (both sides of the fifth and seventh motor nerves). Abbreviations for muscles (so, sr,io, ir, lap,do, am, ima,imp, ah, ao,ih, and hh) are shown in Table 1. *cn is likely to be the ciliary nerve (Fig.6_E, legend_). Scale bar, 100 μm.
Fig. 8.
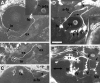
Position of the trigeminal motor (nV) neurons with respect to the peripheral nerve branches.A, A diagram showing dye application sites and a summary of the results. Trigeminal motor neurons were retrogradely labeled with DiI. DiI application was performed at positions 1–5. The results showed that Va solely consists of neurons projecting to the intermediate branch (asterisk), whereas Vp consists of neurons projecting to the either proximal (circle) or distal branch (square). Abbreviations for the muscles are the same as those shown in Table 1. In B–J, all pictures are dorsal views (composite pictures made from stacked confocal images) of larvae at ∼90 hr with anterior to the_left_ and medial to the top.B–D, DiI was applied at position 1. Neurons belonging to the posterior cluster (Vp) of the nV neurons were labeled with DiI. E–G, DiI was applied at position 3. Neurons belonging to the anterior cluster (Va) were labeled with DiI. H–J, DiI was applied at position 4. Neurons belonging to the posterior cluster (Vp) were labeled. Scale bar, 20 μm.
Fig. 9.
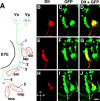
Mosaic analysis of the Isl1-GFP line. Mosaic animals were made by isochronically transplanting cells of the Isl1-GFP line into the normal embryo at the sphere stage to obtain stochastic labeling of cranial motor and sensory neurons. All images except for C are taken from larvae at ∼75 hr.C is from a larva at ∼90 hr. All figures are composite pictures made from the stacked confocal images. A,Lateral (slightly ventral) view of a mosaic larva in which GFP is expressed in the facial sensory ganglion cells (fs). B, C, Ventral (B) and dorsal (C) views of the same larva shown in A. a-fs shows the centrally projecting afferent facial sensory axons, which terminate in the dorsoposterior part of the hindbrain (arrowhead). The asterisk indicates signals derived from GFP-expressing cells in the mesencephalic region (Fig. 7_A, a; B, d; see also single and double asterisks in E). Peripherally extending axons from the facial sensory ganglion cells take internal (in) and external (ex) pathways. Axons of both internal and external pathways terminate near the mouth. The internal fibers (correspond to a in Fig.6_C) take a deep route, and most of the parts of the fibers are not visible, partly because they run out of the focal plane, and partly because of the opaque nature of the eye (lateral view) and cartilages (ventral view). The external fibers initially take the same superficial pathway as the seventh motor nerve and then diverge (Fig.6_C_, b; D,E, 7_E, arrows_). D, Lateral view of a mosaic larva in which GFP is expressed in neurons in the facial sensory ganglion (fs), in the glossopharyngeal sensory ganglion (gs), and the vagus sensory ganglion (short arrows). a-fs shows the centrally projecting afferent facial sensory axons, which terminate in the dorsoposterior part of the hindbrain (arrowhead). The_long thin arrow_ indicates the faintly fluorescent centrally projecting axons from the glossopharyngeal sensory neurons. Terminals of the centrally projecting axons from the facial, glossopharyngeal, and vagus ganglion cells are located near one another in the dorsoposterior part of the hindbrain. The terminal region is likely to correspond to the nucleus tractus solitarii in the adult fish. We have examined a number of mosaic embryos in which GFP was expressed in the facial sensory neurons (n > 15). In all cases, centrally projecting fibers took the same pathway (Figs.6_A_,E, 7_A_,a-fs; 6_C, green_), and peripherally projecting fibers eventually terminated around the mouth.E, Dorsal view of a mosaic embryo in which GFP is expressed in a number of cells other than the motor neurons in the brain. The double-headed arrow marks the midline. The_double asterisks_ and the single asterisk_indicate the GFP-positive cells in the mesencephalic region (Fig.7_A, a; B, d). Anteriorly projecting processes from these cells (probably from the cells marked by the double asterisk) correspond to the tracts marked by c_in Figure 7_B, whereas posteriorly projecting processes from these cells (probably from the cells marked by the single asterisk) correspond to the tracts marked by e_in Figure 7_B. The posteriorly projecting axons may terminate around the trigeminal motor nucleus and facial motor nucleus (arrows). Scale bar, 100 μm.
Similar articles
- Functional repression of Islet-2 by disruption of complex with Ldb impairs peripheral axonal outgrowth in embryonic zebrafish.
Segawa H, Miyashita T, Hirate Y, Higashijima S, Chino N, Uyemura K, Kikuchi Y, Okamoto H. Segawa H, et al. Neuron. 2001 May;30(2):423-36. doi: 10.1016/s0896-6273(01)00283-5. Neuron. 2001. PMID: 11395004 - Role of branchiomotor neurons in controlling food intake of zebrafish larvae.
Allen JR, Bhattacharyya KD, Asante E, Almadi B, Schafer K, Davis J, Cox J, Voigt M, Viator JA, Chandrasekhar A. Allen JR, et al. J Neurogenet. 2017 Sep;31(3):128-137. doi: 10.1080/01677063.2017.1358270. Epub 2017 Aug 16. J Neurogenet. 2017. PMID: 28812416 Free PMC article. - High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin.
Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. Higashijima S, et al. Dev Biol. 1997 Dec 15;192(2):289-99. doi: 10.1006/dbio.1997.8779. Dev Biol. 1997. PMID: 9441668 - [Study of mechanisms for the axonal pathfinding of the trigeminal motor neurons by using transgenic zebrafish].
Wada H, Okamoto H, Higashijima S. Wada H, et al. Tanpakushitsu Kakusan Koso. 2000 Dec;45(17 Suppl):2803-9. Tanpakushitsu Kakusan Koso. 2000. PMID: 11187783 Review. Japanese. No abstract available. - [Developmental regulation of sensory and motor neurons by LIM/homeodomain-transcription factors].
Segawa H, Okamoto H. Segawa H, et al. Tanpakushitsu Kakusan Koso. 2000 Dec;45(17 Suppl):2791-7. Tanpakushitsu Kakusan Koso. 2000. PMID: 11187781 Review. Japanese. No abstract available.
Cited by
- Mammalian genome research resources available from the National BioResource Project in Japan.
Mizuno-Iijima S, Kawamoto S, Asano M, Mashimo T, Wakana S, Nakamura K, Nishijima KI, Okamoto H, Saito K, Yoshina S, Miwa Y, Nakamura Y, Ohkuma M, Yoshiki A. Mizuno-Iijima S, et al. Mamm Genome. 2024 Dec;35(4):497-523. doi: 10.1007/s00335-024-10063-2. Epub 2024 Sep 11. Mamm Genome. 2024. PMID: 39261329 Free PMC article. Review. - Comparative distribution and in vitro activities of the urotensin II-related peptides URP1 and URP2 in zebrafish: evidence for their colocalization in spinal cerebrospinal fluid-contacting neurons.
Quan FB, Dubessy C, Galant S, Kenigfest NB, Djenoune L, Leprince J, Wyart C, Lihrmann I, Tostivint H. Quan FB, et al. PLoS One. 2015 Mar 17;10(3):e0119290. doi: 10.1371/journal.pone.0119290. eCollection 2015. PLoS One. 2015. PMID: 25781313 Free PMC article. - In vivo trafficking and targeting of N-cadherin to nascent presynaptic terminals.
Jontes JD, Emond MR, Smith SJ. Jontes JD, et al. J Neurosci. 2004 Oct 13;24(41):9027-34. doi: 10.1523/JNEUROSCI.5399-04.2004. J Neurosci. 2004. PMID: 15483121 Free PMC article. - Nicotinic receptors mediate changes in spinal motoneuron development and axonal pathfinding in embryonic zebrafish exposed to nicotine.
Svoboda KR, Vijayaraghavan S, Tanguay RL. Svoboda KR, et al. J Neurosci. 2002 Dec 15;22(24):10731-41. doi: 10.1523/JNEUROSCI.22-24-10731.2002. J Neurosci. 2002. PMID: 12486166 Free PMC article. - WldS and PGC-1α regulate mitochondrial transport and oxidation state after axonal injury.
O'Donnell KC, Vargas ME, Sagasti A. O'Donnell KC, et al. J Neurosci. 2013 Sep 11;33(37):14778-90. doi: 10.1523/JNEUROSCI.1331-13.2013. J Neurosci. 2013. PMID: 24027278 Free PMC article.
References
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. - PubMed
- Burrill JD, Easter S., Jr Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio). J Comp Neurol. 1994;346:583–600. - PubMed
- Chandrasekhar A, Moens CB, Warren J, Jr, Kimmel CB, Kuwada JY. Development of branchiomotor neurons in zebrafish. Development. 1997;124:2633–2644. - PubMed
- Chandrasekhar A, Warren JT, Jr, Takahashi K, Schauerte HE, van Eeden FJ, Haffter P, Kuwada JY. Role of sonic hedgehog in branchiomotor neuron induction in zebrafish. Mech Dev. 1998;76:101–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases