HuR regulates p21 mRNA stabilization by UV light - PubMed (original) (raw)
HuR regulates p21 mRNA stabilization by UV light
W Wang et al. Mol Cell Biol. 2000 Feb.
Abstract
Expression of the cyclin-dependent kinase inhibitor p21 is highly induced by many stresses, including exposure to short-wavelength UV light (UVC), which increases p21 mRNA stability. Investigation into the mechanisms underlying this stabilization process revealed that proteins present in cytoplasmic lysates of human RKO colorectal carcinoma cells formed complexes with p21 mRNA that were inducible by treatment with UVC and other stress agents. The ubiquitous Elav-type RNA-binding protein HuR was identified within the p21 mRNA-protein complexes, as antibodies recognizing HuR supershifted these complexes and revealed HuR-immunoreactive proteins complexing with p21 mRNA on Western blots. Lowering of endogenous HuR levels through expression of antisense HuR decreased p21 RNA-protein complexes, greatly reduced the UVC inducibility and half-life of p21 mRNA, and prevented UVC-mediated induction of luciferase activity in p21 3' untranslated region-containing reporter constructs. Our findings indicate that HuR plays a major role in regulating stress-induced p21 expression by enhancing p21 mRNA stability and that these effects are coupled to HuR's elevated presence in the cytoplasm.
Figures
FIG. 1
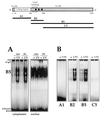
Structure of the p21 mRNA and fragment analysis. Top, structure of the full-length p21 mRNA, indicating the coding and untranslated regions (5′ and 3′ UTRs). Squares represent AUUUA sequences; the region previously shown to bind HuD (30) is indicated. Heavy lines indicate the RNAs used in this study. Corresponding PCR-amplified regions served as templates for in vitro transcription of these RNA molecules (see Materials and Methods). (A) B5 was incubated with cytoplasmic and nuclear lysates of RKO cells (neo and E6) 6 h following treatment with UVC (20 J/m2; UV) or no treatment (−); unbound transcript was digested with RNase T1. Reaction products were resolved by electrophoresis through native 7% polyacrylamide gels. (B) Cytoplasmic lysates from either untreated or UVC-treated cells were tested for binding to the various transcripts shown in panel A. Binding assays and electrophoresis were carried out as described in Materials and Methods.
FIG. 2
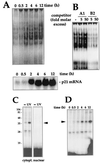
Characterization of binding activity: time course, specificity, and cross-linking assays. (A) After UVC irradiation of RKO cells, cytoplasmic lysates were prepared at the times indicated and assayed for binding to B2 (top), or total mRNA, prepared and p21 mRNA expression assayed by Northern blotting (bottom); RNA was loaded evenly, as assessed after stripping of membranes and hybridization using a probe recognizing 18S (not shown). (B) Binding specificity was tested in cytoplasmic lysates from UVC-treated cells and B5, in the absence (−) or presence of the indicated molar excesses of unlabeled B2 or A1 RNAs. (C) Subcellular fractions were incubated with B2, digested with RNase T1, cross-linked, and resolved by SDS-PAGE (15% gel). Gels were dried and exposed to X-ray film to visualize radiolabeled complexes. Numbers denote sizes (in kilodaltons) of molecular weight markers. (D) At the times indicated after UVC irradiation, cytoplasmic lysates were prepared and binding to B2 was assayed. Processing of samples was carried out as described for panel A. Arrowheads indicate inducible complexes.
FIG. 3
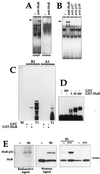
HuR binds the p21 mRNA in vivo and in vitro. (A) Monoclonal antibody 19F12 (4 μg) was incubated with cytoplasmic or nuclear lysates of UVC-treated cells and B2. Complexes were resolved in native 7% polyacrylamide gels. (B) The indicated antibodies were tested for their ability to supershift complexes forming between cytoplasmic proteins and B2. Arrowheads indicate positions of specific supershifted complexes. (C) RNase T1 selection assay was carried out with B2 and A1, incubated with 10 nM GST or GST-HuR (see Materials and Methods). T1, digestions with RNase T1 alone; M, molecular weight markers. (D) Gel retardation assays using B2 and the indicated concentrations of either GST or GST-HuR. (E) Left, cytoplasmic fractions were either incubated with B2 or not, cross-linked, digested with RNase T1, resolved by SDS-PAGE (15% gel), and transferred onto polyvinylidene difluoride membranes, which were sequentially exposed to X-ray film for 24 h (Radioactive signal) and subjected to Western blot analysis to detect HuR (Western signal); exposure time, 30 s. Right, Lysates from UVC-treated or untreated cells were incubated with B2 and then subjected to Western blot analysis. Estimated size of the HuR-p21 complexes, 37 to 40 kDa.
FIG. 4
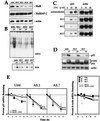
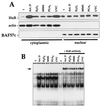
Decreased HuR expression lowers binding to the p21 3′ UTR and reduces p21 mRNA stability and p21 induction by UVC. (A) Western blot analysis of HuR expression in RKO cells, either untransfected (untr.) or transfected with pZeoSV2(−)HuR, expressing AS HuR. Chosen clonal isolates are shown. Blots were sequentially stripped and rehybridized with an antibody recognizing actin (43 kDa), to visualize differences in loading and transfer, and with an antibody recognizing hnRNP C (43 kDa). (B) B5 binding activity in lysates from untransfected and AS HuR-expressing cells 6 h after UVC irradiation. (C) Northern blot analysis of p21 mRNA expression in untransfected and AS HuR-expressing RKO cells 8 h after either no treatment (−) or exposure to the indicated UVC doses. Evenness in loading and transfer among samples was assessed after stripping the membrane and rehybridizing it with an oligomer probe recognizing 18S rRNA. (D) Western blot analysis to assess the expression of p21, c-Jun (39 kDa), and actin in untransfected and AS HuR-expressing RKO cells 10 h after either no treatment or exposure to 20 J/m2 UVC. p-jun, phosphorylated Jun. (E) Graphs depict the rate of loss of p21 and β-actin mRNAs in cells with different HuR levels after actinomycin D (2 μg/ml) addition with or without UVC irradiation. At the times indicated, total RNA was extracted and p21 and β-actin mRNAs were monitored by Northern blotting; signals were quantitated with a PhosphorImager, normalized against 18S (not shown), and plotted on a logarithmic scale. The mRNA half-life in each treatment group is indicated in parentheses. Values represent means ± standard errors of the means of three independent experiments.
FIG. 5
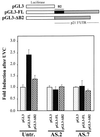
Effect of the full-length and mutant p21 3′ UTR on expression of a luciferase reporter construct. (Top) Expression vectors pGL3, pGL3-FL, and pGL3-ΔB2 (see Materials and Methods) were transiently cotransfected into RKO parental (untransfected [Untr.]), AS.2, or AS.7 cells along with pSV-βgal (used to normalize for transfection efficiency); cells were irradiated with UVC (20 J/m2) or left untreated, and luciferase and β-galactosidase activities were examined 24 h later. (Bottom) Relative fold increase in luciferase activity after UVC exposure, seen with either pGL3-FL or pGL3-ΔB2 compared with that seen with the control vector pGL3. Values represent means ± standard errors of the means of five independent experiments.
FIG. 6
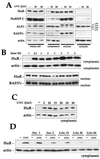
Western blot analysis of HuR expression and subcellular localization. (A) Six hours after irradiation with the indicated doses of UVC, whole-cell (20 μg), cytoplasmic (40 μg), nuclear (10 μg), and cytosolic (40 μg) lysates were prepared and subjected to Western blot analysis to monitor the expression of HuR, hnRNP C, AUF1, BAF57c (57 kDa), and actin. Cell lysates were collected at the times indicated after irradiation with UVC (20 J/m2) (B) or 6 h after irradiation with the indicated doses of UVC (C), and Western blot analysis of HuR expression performed on cytoplasmic (40 μg) and nuclear (10 μg) fractions. (D) Indicated doses of ionomycin (Ion.; micromolar) or lithium acetate (LiAc; millimolar) were added to cells 1 h before UVC irradiation with 20 J/m2 and Western blot analysis of cytoplasmic HuR. Hybridization using antibodies against actin and BAF57c was carried out to assess uniformity in loading and transfer among cytoplasmic and nuclear samples, respectively.
FIG. 7
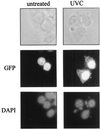
Subcellular localization of HuR. GFP-HuR was visualized by fluorescence microscopy in transiently transfected RKO cells that were either left untreated or treated with 20 J of UVC/m2 (4 h earlier). DAPI staining served to visualize the nucleus. Note the distinct overlap of DAPI and GFP-HuR signals in untreated cells; while UVC-irradiated cells also exhibit abundant nuclear GFP-HuR, the treatment causes a substantial increase in the cytoplasmic GFP-HuR signal, not seen in untreated cells.
FIG. 8
Increased cytoplasmic HuR and p21 RNA binding after exposure to stresses. (A) Western blot analysis to monitor HuR expression in cytoplasmic and nuclear fractions after treatment with the indicated agents. Samples were collected 2 h after addition of actinomycin (Act.) D (1 μg/ml) or 4 h after exposure to 100 μM H2O2, MMS (100 μg/ml), 48 μM PGA2, or UVC (20 J/m2). Hybridizations using antibodies against actin and BAF57c were carried out to assess uniformity in loading and transfer among cytoplasmic and nuclear samples, respectively. (B) B2 binding activity in cytoplasmic lysates of cells treated as for panel and supershift analysis of complexes forming after exposure to such stresses.
Similar articles
- Prostaglandin A2-mediated stabilization of p21 mRNA through an ERK-dependent pathway requiring the RNA-binding protein HuR.
Yang X, Wang W, Fan J, Lal A, Yang D, Cheng H, Gorospe M. Yang X, et al. J Biol Chem. 2004 Nov 19;279(47):49298-306. doi: 10.1074/jbc.M407535200. Epub 2004 Sep 14. J Biol Chem. 2004. PMID: 15371446 - The 3'-untranslated region of p21WAF1 mRNA is a composite cis-acting sequence bound by RNA-binding proteins from breast cancer cells, including HuR and poly(C)-binding protein.
Giles KM, Daly JM, Beveridge DJ, Thomson AM, Voon DC, Furneaux HM, Jazayeri JA, Leedman PJ. Giles KM, et al. J Biol Chem. 2003 Jan 31;278(5):2937-46. doi: 10.1074/jbc.M208439200. Epub 2002 Nov 12. J Biol Chem. 2003. PMID: 12431987 - HuR, a key post-transcriptional regulator, and its implication in progression of breast cancer.
Yuan Z, Sanders AJ, Ye L, Jiang WG. Yuan Z, et al. Histol Histopathol. 2010 Oct;25(10):1331-40. doi: 10.14670/HH-25.1331. Histol Histopathol. 2010. PMID: 20712017 Review. - Targeting the RNA-Binding Protein HuR in Cancer.
Finan JM, Sutton TL, Dixon DA, Brody JR. Finan JM, et al. Cancer Res. 2023 Nov 1;83(21):3507-3516. doi: 10.1158/0008-5472.CAN-23-0972. Cancer Res. 2023. PMID: 37683260 Review.
Cited by
- MicroRNAs in skin response to UV radiation.
Syed DN, Khan MI, Shabbir M, Mukhtar H. Syed DN, et al. Curr Drug Targets. 2013 Sep;14(10):1128-34. doi: 10.2174/13894501113149990184. Curr Drug Targets. 2013. PMID: 23834148 Free PMC article. Review. - Upregulation of the host SLC11A1 gene by Clostridium difficile toxin B facilitates glucosylation of Rho GTPases and enhances toxin lethality.
Feng Y, Cohen SN. Feng Y, et al. Infect Immun. 2013 Aug;81(8):2724-32. doi: 10.1128/IAI.01177-12. Epub 2013 May 20. Infect Immun. 2013. PMID: 23690404 Free PMC article. - Insights from the HuR-interacting transcriptome: ncRNAs, ubiquitin pathways, and patterns of secondary structure dependent RNA interactions.
St Laurent G 3rd, Shtokalo D, Heydarian M, Palyanov A, Babiy D, Zhou J, Kumar A, Urcuqui-Inchima S. St Laurent G 3rd, et al. Mol Genet Genomics. 2012 Dec;287(11-12):867-79. doi: 10.1007/s00438-012-0722-8. Epub 2012 Oct 4. Mol Genet Genomics. 2012. PMID: 23052832 - A role for both Ets and C/EBP transcription factors and mRNA stabilization in the MAPK-dependent increase in p21 (Cip-1/WAF1/mda6) protein levels in primary hepatocytes.
Park JS, Qiao L, Gilfor D, Yang MY, Hylemon PB, Benz C, Darlington G, Firestone G, Fisher PB, Dent P. Park JS, et al. Mol Biol Cell. 2000 Sep;11(9):2915-32. doi: 10.1091/mbc.11.9.2915. Mol Biol Cell. 2000. PMID: 10982390 Free PMC article. - The ARE-dependent mRNA-destabilizing activity of BRF1 is regulated by protein kinase B.
Schmidlin M, Lu M, Leuenberger SA, Stoecklin G, Mallaun M, Gross B, Gherzi R, Hess D, Hemmings BA, Moroni C. Schmidlin M, et al. EMBO J. 2004 Dec 8;23(24):4760-9. doi: 10.1038/sj.emboj.7600477. Epub 2004 Nov 11. EMBO J. 2004. PMID: 15538381 Free PMC article.
References
- Atasoy U, Watson J, Patel D, Keene J D. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J Cell Sci. 1998;111:3145–3156. - PubMed
- Badminton M N, Kendall J M, Rembold C M, Campbell A K. Current evidence suggests independent regulation of nuclear calcium. Cell Calcium. 1998;23:79–86. - PubMed
- Barami K, Iversen K, Furneaux H, Goldman S. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28:82–101. - PubMed
- Bellido T, O'Brien C A, Roberson P K, Manolagas S C. Transcriptional activation of the p21WAF1, CIP1, SDI1 gene by interleukin-6 type cytokines. A prerequisite for their pro-differentiating and anti-apoptotic effects on human osteoblastic cells. J Biol Chem. 1998;273:21137–21144. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous