Exonic splicing enhancer motif recognized by human SC35 under splicing conditions - PubMed (original) (raw)
Exonic splicing enhancer motif recognized by human SC35 under splicing conditions
H X Liu et al. Mol Cell Biol. 2000 Feb.
Abstract
Exonic splicing enhancers (ESEs) are important cis elements required for exon inclusion. Using an in vitro functional selection and amplification procedure, we have identified a novel ESE motif recognized by the human SR protein SC35 under splicing conditions. The selected sequences are functional and specific: they promote splicing in nuclear extract or in S100 extract complemented by SC35 but not by SF2/ASF. They can also function in a different exonic context from the one used for the selection procedure. The selected sequences share one or two close matches to a short and highly degenerate octamer consensus, GRYYcSYR. A score matrix was generated from the selected sequences according to the nucleotide frequency at each position of their best match to the consensus motif. The SC35 score matrix, along with our previously reported SF2/ASF score matrix, was used to search the sequences of two well-characterized splicing substrates derived from the mouse immunoglobulin M (IgM) and human immunodeficiency virus tat genes. Multiple SC35 high-score motifs, but only two widely separated SF2/ASF motifs, were found in the IgM C4 exon, which can be spliced in S100 extract complemented by SC35. In contrast, multiple high-score motifs for both SF2/ASF and SC35 were found in a variant of the Tat T3 exon (lacking an SC35-specific silencer) whose splicing can be complemented by either SF2/ASF or SC35. The motif score matrix can help locate SC35-specific enhancers in natural exon sequences.
Figures
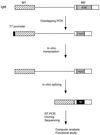
FIG. 1
Experimental procedure for functional SELEX. The structure of the IgM M1-M2 minigene pre-mRNA is shown. The characterized natural ESE (73 nt) was replaced by 20 nt of randomized sequence by overlap-extension PCR (20). A T7 promoter (black box) was built into the PCR product. In vitro-transcribed RNA was then incubated under splicing conditions. Any spliced mRNA molecules must contain a functional ESE or winner sequence, designated by the white W in a black box. The spliced mRNA molecules were purified from a denaturing polyacrylamide gel, reamplified by RT-PCR, and cloned. Individual clones were sequenced and analyzed by a sampling algorithm to define a common motif, and a subset was rebuilt into minigene templates, transcribed, and assayed for splicing in vitro.
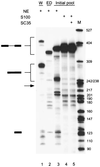
FIG. 2
Splicing of the initial RNA pool. Twenty femtomoles of wild-type IgM minigene pre-mRNA (W; lane 1), pre-mRNA with a deletion of the ESE (ED; lane 2), or a pre-mRNA pool representing 1.2 × 1010 different molecules with a randomized 20-nt segment within exon M2 were spliced in 25-μl reaction mixtures in nuclear extract (lane 3), S100 extract alone (lane 4), or S100 extract complemented by 20 pmol of recombinant SC35 (lane 5). The structures of the precursor, intermediates, and products are indicated next to the autoradiogram. The expected size of the spliced mRNA product of the ESE deletion mutant is indicated by an arrow.
FIG. 3
Analysis of the SC35-selected sequences. (A) Sequence alignment and identification of a consensus motif. The consensus motif and score matrix were derived as described previously (20). The sequences were aligned on the basis of the highest score motif for each sequence. Nucleotides matching the consensus are shown as white on a black background; mismatched nucleotides are not shaded. The scores of the aligned motifs are indicated on the right. Additional motifs present in some of the sequences with a score greater than 1.62 (the mean score of the random pool) are underlined. In two cases these include a trinucleotide contributed by the 3′-flanking sequence, which is indicated with lowercase letters (cua). The consensus shown is only an approximation that indicates the most frequent nucleotide(s) at each position. The lowercase c at position 5 denotes a slight preference for this nucleotide over the other three nucleotides, which occur at similar frequencies. Y, pyrimidine; S, G or C; R, purine. The nucleotide composition of the selected pool is shown at the bottom. The nucleotide composition of the initial RNA pool was as follows: A, 21%; G, 39%; C, 19%, and U, 21%. (B) Representation of the SC35 ESE score matrix and consensus motif. The diagram shows the frequency of each nucleotide at each position of the octamer consensus, adjusted for the compositional bias of the initial pool (20). The height of each letter is proportional to its frequency, and the nucleotides are shown from top to bottom in decreasing order of frequency. This method of displaying nucleotide frequencies is based on references (3) and (13).
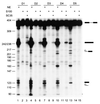
FIG. 4
Activity of the selected ESE motifs. SC35 SELEX winners were rebuilt into the IgM M1-M2 minigene by overlap-extension PCR (as described in the legend for Fig. 1), and transcripts corresponding to individual winners were spliced in HeLa nuclear extract (lanes 1, 4, 7, 10, and 13), in S100 extract alone (lanes 2, 5, 8, 11, and 14), or in S100 extract complemented by recombinant SC35 (lanes 3, 6, 9, 12, and 15).
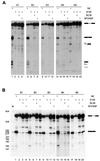
FIG. 5
Specificity of the selected ESE motifs. (A) Splicing of SC35-selected ESEs was analyzed in nuclear extract (lanes 1, 5, 9, 13, and 17), S100 extract alone (lanes 2, 6, 10, 14, and 17), or S100 extract plus recombinant SC35 (lanes 3, 7, 11, 15, and 19) or recombinant SF2/ASF (lanes 4, 8, 12, 16, and 20). (B) Splicing of SF2/ASF-selected ESEs was examined in nuclear extract (lanes 1, 5, 9, 13, and 17), S100 extract alone (lanes 2, 6, 10, 14, and 18), or in S100 extract plus SC35 (lanes 3, 7, 11, 15, and 19) or SF2/ASF (lanes 4, 8, 12, 16, and 20).
FIG. 6
Comparison of SC35 winner sequences and SC35 ESE motif in two different exonic contexts. (A) The 20-nt D2 winner sequence (see Fig. 3), a variant of D2 with two nucleotide changes to introduce a maximum-score consensus (D2C), and a 19-nt SC35 SELEX winner sequence (6-24) described in a previous study (29) were inserted into the IgM M1-M2 minigene in place of the natural ESE in exon M2, and the corresponding transcripts were spliced in nuclear extract (lanes 2 to 4). The control pre-mRNA lacking an ESE (ED) is shown in lane 1. (B) The same D2, D2C, and 6-24 sequences were tested in the context of an IgM C3-C4 minigene (26). The Ca pre-mRNA includes the first 38 nt of the C4 exon (lane 1). In the remaining pre-mRNAs, this segment of the C4 exon is followed by the next 38 nt of the C4 exon, which comprise a natural SC35-dependent ESE (CaCb; lane 2) or it is followed by the D2, D2C, or 6-24 sequence (lanes 3 to 5). The sequences of the relevant portions of the 3′ exons are shown below each panel. The two nucleotide changes in D2C, compared to D2, are underlined. The mobilities of the pre-mRNAs, mRNAs, and 5′ exon intermediates are indicated next to each autoradiogram.
FIG. 7
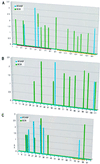
Correlation between predicted ESE motifs in natural genes and SR protein specificity of the pre-mRNAs. Score matrices derived for SC35 and SF2/ASF were used to search the sequences of natural genes. The resulting scores (y axis) were plotted against the nucleotide position along each exon (x axis). The vertical bars indicate the first nucleotide of each motif. SC35 high-score motifs are shown in green, and SF2/ASF ones are shown in blue. Since different score matrices were used for each protein, the numerical scores of the two different proteins cannot be compared. (A) High-score motifs in the IgM gene M2 exon. The characterized ESE is indicated by the horizontal magenta bar, and the yellow bar indicates the region comprising a recently described silencer (15, 39). (B) High-score motifs in the IgM gene C4 exon. (C) High-score motifs in the tat gene T3 exon. The horizontal yellow bar indicates the position of the SC35-specific silencer.
Similar articles
- hnRNP A1 and the SR proteins ASF/SF2 and SC35 have antagonistic functions in splicing of beta-tropomyosin exon 6B.
Expert-Bezançon A, Sureau A, Durosay P, Salesse R, Groeneveld H, Lecaer JP, Marie J. Expert-Bezançon A, et al. J Biol Chem. 2004 Sep 10;279(37):38249-59. doi: 10.1074/jbc.M405377200. Epub 2004 Jun 18. J Biol Chem. 2004. PMID: 15208309 - Substrate specificities of SR proteins in constitutive splicing are determined by their RNA recognition motifs and composite pre-mRNA exonic elements.
Mayeda A, Screaton GR, Chandler SD, Fu XD, Krainer AR. Mayeda A, et al. Mol Cell Biol. 1999 Mar;19(3):1853-63. doi: 10.1128/MCB.19.3.1853. Mol Cell Biol. 1999. PMID: 10022872 Free PMC article. - Exon identity established through differential antagonism between exonic splicing silencer-bound hnRNP A1 and enhancer-bound SR proteins.
Zhu J, Mayeda A, Krainer AR. Zhu J, et al. Mol Cell. 2001 Dec;8(6):1351-61. doi: 10.1016/s1097-2765(01)00409-9. Mol Cell. 2001. PMID: 11779509 - The human splicing factors ASF/SF2 and SC35 possess distinct, functionally significant RNA binding specificities.
Tacke R, Manley JL. Tacke R, et al. EMBO J. 1995 Jul 17;14(14):3540-51. doi: 10.1002/j.1460-2075.1995.tb07360.x. EMBO J. 1995. PMID: 7543047 Free PMC article. - Human papillomavirus regulation of SR proteins.
McFarlane M, Graham SV. McFarlane M, et al. Biochem Soc Trans. 2010 Aug;38(4):1116-21. doi: 10.1042/BST0381116. Biochem Soc Trans. 2010. PMID: 20659014 Review.
Cited by
- Molecular impact of mutations in RNA splicing factors in cancer.
Zhang Q, Ai Y, Abdel-Wahab O. Zhang Q, et al. Mol Cell. 2024 Oct 3;84(19):3667-3680. doi: 10.1016/j.molcel.2024.07.019. Epub 2024 Aug 14. Mol Cell. 2024. PMID: 39146933 Review. - The U1-70K and SRSF1 interaction is modulated by phosphorylation during the early stages of spliceosome assembly.
Paul T, Zhang P, Zhang Z, Fargason T, De Silva NIU, Powell E, Ekpenyong E, Jamal S, Yu Y, Prevelige P, Lu R, Zhang J. Paul T, et al. Protein Sci. 2024 Aug;33(8):e5117. doi: 10.1002/pro.5117. Protein Sci. 2024. PMID: 39023093 Free PMC article. - circSMARCA5 Is an Upstream Regulator of the Expression of miR-126-3p, miR-515-5p, and Their mRNA Targets, Insulin-like Growth Factor Binding Protein 2 (IGFBP2) and NRAS Proto-Oncogene, GTPase (NRAS) in Glioblastoma.
Merulla AE, Stella M, Barbagallo C, Battaglia R, Caponnetto A, Broggi G, Altieri R, Certo F, Caltabiano R, Ragusa M, Barbagallo GMV, Di Pietro C, Purrello M, Barbagallo D. Merulla AE, et al. Int J Mol Sci. 2022 Nov 8;23(22):13676. doi: 10.3390/ijms232213676. Int J Mol Sci. 2022. PMID: 36430152 Free PMC article. - Alternative splicing of HSPA12A pre-RNA by SRSF11 contributes to metastasis potential of colorectal cancer.
Pan YJ, Huo FC, Kang MJ, Liu BW, Wu MD, Pei DS. Pan YJ, et al. Clin Transl Med. 2022 Nov;12(11):e1113. doi: 10.1002/ctm2.1113. Clin Transl Med. 2022. PMID: 36394206 Free PMC article. - Human PRPF39 is an alternative splicing factor recruiting U1 snRNP to weak 5' splice sites.
Espinosa S, De Bortoli F, Li X, Rossi J, Wagley ME, Lo HG, Taliaferro JM, Zhao R. Espinosa S, et al. RNA. 2022 Oct 31;29(1):97-110. doi: 10.1261/rna.079320.122. Online ahead of print. RNA. 2022. PMID: 36316087 Free PMC article.
References
- Berget S M. Exon recognition in vertebrate splicing. J Biol Chem. 1995;270:2411–2414. - PubMed
- Burge C B, Tuschl T, Sharp P A. Splicing of precursors to mRNAs by the spliceosomes. In: Gesteland R F, Cech T R, Atkins J F, editors. The RNA world. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1999. pp. 525–559.
- Burset M, Guigo R. Evaluation of gene structure prediction programs. Genomics. 1996;34:353–367. - PubMed
- Cáceres J F, Stamm S, Helfman D M, Krainer A R. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–1709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM042699/GM/NIGMS NIH HHS/United States
- HG01696/HG/NHGRI NIH HHS/United States
- R01 GM042699/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- GM42699/GM/NIGMS NIH HHS/United States
- R01 HG001696/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous