Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering - PubMed (original) (raw)
Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering
A E El-Husseini et al. J Cell Biol. 2000.
Abstract
Postsynaptic density-95 (PSD-95/SAP-90) is a palmitoylated peripheral membrane protein that scaffolds ion channels at excitatory synapses. To elucidate mechanisms for postsynaptic ion channel clustering, we analyzed the cellular trafficking of PSD-95. We find that PSD-95 transiently associates with a perinuclear membranous compartment and traffics with vesiculotubular structures, which migrate in a microtubule-dependent manner. Trafficking of PSD-95 with these vesiculotubular structures requires dual palmitoylation, which is specified by five consecutive hydrophobic residues at the NH(2) terminus. Mutations that disrupt dual palmitoylation of PSD-95 block both ion channel clustering by PSD-95 and its synaptic targeting. Replacing the palmitoylated NH(2) terminus of PSD-95 with alternative palmitoylation motifs at either the NH(2) or COOH termini restores ion channel clustering also induces postsynaptic targeting, respectively. In brain, we find that PSD-95 occurs not only at PSDs but also in association with intracellular smooth tubular structures in dendrites and spines. These data imply that PSD-95 is an itinerant vesicular protein; initial targeting of PSD-95 to an intracellular membrane compartment may participate in postsynaptic ion channel clustering by PSD-95.
Figures
Figure 1
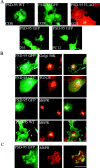
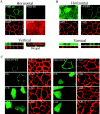
PSD-95 is sorted to a vesicular perinuclear domain. (A) Exogenous PSD-95 accumulates at a perinuclear domain in transfected COS (top), HEK-293 and PC12 cells (bottom). Cells were transfected with either wild-type PSD-95 (PSD-95 WT) or PSD-95 fused to GFP (PSD-95 GFP) or FLAG-epitope tag (PSD-95 FLAG). (B) In COS cells, perinuclear staining for PSD-95 (green) partially overlaps with a Golgi marker Golgi 58K (red) and with a trans-Golgi marker TGN-38 (red), but extensively colocalizes with a late endosomal marker, mannose-6-phosphate receptor (red; M6PR). (C) High magnification shows colocalization of PSD-95 with M6PR. Merged images are shown in the right panels for B and C. Bars, 10 μm.
Figure 2
Accumulation of PSD-95 at a perinuclear domain precedes dendritic clustering in primary hippocampal neurons. Endogenous PSD-95 expression in developing hippocampal neurons is shown at 1 d (DIV1) and 4 d (DIV4) in vitro. The expression pattern of PSD-95 (green) is compared with the endosomal marker M6PR (red) and merged images are shown in the right panels. At DIV1, PSD-95 shows strong perinuclear staining that coincides with M6PR. NMDA receptor subunits, NR1 and NR2B, colocalize with PSD-95 and M6PR at DIV1 whereas MAP2 shows diffuse staining in the soma and processes. By DIV4, PSD-95 occurs diffusely in the soma, and at this stage clusters begin to form in the neurites (arrowheads). Bar, 10 μm.
Figure 3
Perinuclear accumulation of PSD-95 relies on the palmitoylation motif and requires a functional secretory pathway and intact microtubules. (A) COS cells were transfected with PSD-95. 2 h after transfection, cells treated for 7 h with either vehicle or 10 μg/ml BFA, or 20 μg/ml nocodazole and were examined 8 h later for PSD-95 (green) and M6PR (red). For the nocodazole-treated cells, arrowheads point to the single cell in the field transfected with PSD-95 (bottom). (B) Targeting of PSD-95 to a M6PR-positive perinuclear compartment is mediated by an NH2-terminal palmitoylated motif. Amino acids 1–13 but not 1–9 are sufficient for targeting GFP to the perinuclear, M6PR-positive compartment. Perinuclear accumulation is also observed with GFP-fusion constructs containing amino acids 1–26, 1–46, and 1–64. A chimera containing the first 12 amino acids of GAP-43 fused PSD-95 (GAP/PSD-95) is also sorted to the perinuclear domain. (C) Mutations in the palmitoylation motif dramatically affect perinuclear vesicular sorting. Mutations of Cys3 and/or 5 to Ser (C3,5S, C3S, C5S) or amino acids 6-9 (IVTT) to TEIN (denoted as IVTT*) disrupt vesicular sorting, whereas mutations of amino acids 2, 8, or 9 to Ala (D2A; T8A and T89A) do not. All mutations of amino acid 4 (L4Y, L4A, L4S, and L4D) dramatically alter sorting to the perinuclear domain, with the more hydrophilic changes being more significant. Mutations of amino acids 6 and 7 to hydrophobic ones maintain normal sorting (I6L, V7L), whereas mutating either Cys3 or Cys5 to Leu only partially maintains the perinuclear distribution (C3L, C5L). (D) An enlarged image shows in greater detail the localization of PSD-95 along vesiculotubular structures that resemble Golgi-derived tubules (arrowheads) and also to a perinuclear region (arrows). Bar, 10 μm.
Figure 4
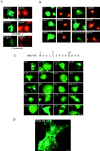
Ultrastructural localization of PSD-95 at synaptic and nonsynaptic sites. The silver-intensified colloidal gold (SIG) method was used for electron micrographic immunolabeling of PSD-95 in visual cortex. (A) Gold particles are associated with the PSD within a spine (arrowhead in Sp1) that opposes an unlabeled terminal, T. Two other spines (Sp2 and Sp3) are without SIG immunolabeling. Within the dendritic shaft (Sh), immunoreactivity is associated with the plasma membrane (curved arrow) and elsewhere. (B) Within a dendritic spine (Sp) of an asymmetric synapse, PSD-95 (dark arrow) is associated with the spine apparatus (three fine arrows). The open arrow points to the unlabeled postsynaptic density. In a dendritic shaft below (Sh), SIG label (curved arrow) is associated with a complex of smooth ER (four fine arrows). Another shaft, to the left of Sp, exhibits immunoreactivity in the cytoplasm, as is shown in A. Bar, 500 nm.
Figure 5
Palmitoylation of PSD-95 and GAP-43 are determined by consensus sequences of 5 consecutive hydrophobic amino acids. (A) Schematic illustration of the domain structure of PSD-95 and sequence alignment of the NH2 terminus of PSD-95 with GAP-43. (B) Analysis of PSD-95 deletion mutants shows that amino acids 1–13 are sufficient for efficient palmitoylation. Mutations of amino acids 6–9 (IVTT to TEIN; IVTT*) have a more dramatic effect on protein palmitoylation than changing amino acids 10–13 (KKYR to NIFS; KKYR*). Insertion of 6 amino acids (VSKSGS) after the starter Met (+6 ins) does not alter palmitoylation. (C) Site-directed mutational analysis of PSD-95 shows that palmitoylation requires hydrophobic amino acids at positions 4, 6, and 7 (see text for detailed analysis). (D) Mutating Cys3 or 5 to Ser blocks palmitoylation whereas changing these residues to Leu partially maintains palmitoylation (C3L, C5L). A chimera containing the first 12 amino acids of GAP-43 fused to PSD-95 (GAP/PSD-95) is efficiently palmitoylated. (E) Hydrophilic, but not hydrophobic, mutations of the residues (Leu-2 and Met-5) surrounding Cys3 and Cys4 of GAP-43 reduce its palmitoylation (see text for detailed analysis).
Figure 6
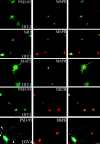
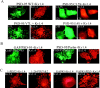
Sorting of PSD-95 to the basolateral membrane of polarized epithelial cells requires protein palmitoylation and PDZ domains. (A) In a horizontal optical section (A, top left pair) stably transfected PSD-95 (green) colocalizes with E-cadherin (CAD, red) along the lateral plasma membranes in polarized MDCK cells. PSD-95 (green) and CAD (red) colocalize along the basolateral membranes in a vertical section (A, bottom left pair). PSD-95 (green) does not occur in an apical horizontal section which is positive for the apical marker gp135 (red). A merged vertical section shows that PSD-95 (green) occurs along the basolateral membranes whereas gp135 (red) is confined to the apical membrane. Cells were investigated by confocal fluorescence microscopy and three-dimensional reconstruction. (B) A stably transfected NH2-terminal mutant of PSD-95 containing all three PDZ domains (1-PDZ3) is sorted to the basolateral membrane whereas a construct extending through only the first PDZ domain (1-PDZ1) occurs diffusely in the cytosol. Horizontal optical sections at the level of the tight junctions and reconstructed vertical sections are shown. (C) Distribution of wild-type and mutants forms of PSD-95, including I6L, I6S, C3L, C5L, L4A, and L4S in transiently transfected MDCK cells. All of the PSD-95 mutant forms, except for I6L, are expressed diffusely in these cells. The mutant form I6L, which maintains palmitoylation, colocalizes with the basolateral marker E-cadherin.
Figure 7
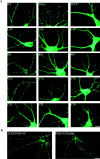
Postsynaptic targeting of PSD-95 in hippocampal neurons requires the NH2-terminal dually palmitoylated motif. (A) Mutations that block PSD-95 palmitoylation (IVTT to TEIN; IVTT*, L4S, I6S) also disrupt postsynaptic targeting of PSD-95. In contrast, mutations that do not disrupt palmitoylation of PSD-95 (I6L, V7L, and T89A) do not alter its postsynaptic targeting. Mutations that reduce palmitoylation (L4Y, L4A, I6A, V7A, and V7S) result in inefficient targeting of PSD-95 to postsynaptic sites. Mutation of Cys3 to Ser (C3S) abolishes protein palmitoylation and synaptic targeting. In contrast, mutations of either Cys to Leu (C3L, C5L), which partially preserve protein palmitoylation, result in very inefficient synaptic targeting. (B) A chimera containing the first 12 amino acids of GAP-43 fused to PSD-95 (GAP/PSD-95) is targeted to postsynaptic sites. The COOH-terminal 12–amino acid palmitoylated motif of paralemmin fused to PSD-95:C3,5S (PSD-95/Parlm) induces postsynaptic clustering of a PSD-95 (C3,5S) mutant. Bar, 10 μm.
Figure 8
Ion channel clustering requires dual palmitoylation of PSD-95. COS cells were cotransfected with Kv1.4 and wild-type or mutant forms of PSD-95 (C3,5S, V7L, or L4S). Cells were fixed 12 h (12h) after transfection and double-labeled with antibodies to PSD-95 (green) and Kv1.4 (red). Palmitoylation-deficient mutants of PSD-95 (C3,5S, or L4S) block clustering whereas the palmitoylated mutant (V7L) functions normally. (B) Chimeras containing the first 12 amino acids of GAP-43 fused to PSD-95 (GAP/PSD-95) or the last 12 amino acids of paralemmin fused to PSD-95 C3,5S (PSD-95/Parlm) mediate clustering of Kv1.4. (C) Cotransfection of Kv1.4 with a construct containing either the first 300 amino acids of PSD-95 (1-PDZ2) or amino acids 1-26 fused to PDZ1&2 (1-26-PDZ1&2) are sufficient for clustering Kv1.4. A construct containing full-length GAP-43 fused to PDZ domains 1 and 2 (GAP43-PDZ) but not GAP-43 itself also induces channel clustering. Bar, 10 μm.
Similar articles
- Ion channel clustering by membrane-associated guanylate kinases. Differential regulation by N-terminal lipid and metal binding motifs.
El-Husseini AE, Topinka JR, Lehrer-Graiwer JE, Firestein BL, Craven SE, Aoki C, Bredt DS. El-Husseini AE, et al. J Biol Chem. 2000 Aug 4;275(31):23904-10. doi: 10.1074/jbc.M909919199. J Biol Chem. 2000. PMID: 10779526 - PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering.
Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. Tiffany AM, et al. J Cell Biol. 2000 Jan 10;148(1):147-58. doi: 10.1083/jcb.148.1.147. J Cell Biol. 2000. PMID: 10629225 Free PMC article. - Neuronal inwardly rectifying K(+) channels differentially couple to PDZ proteins of the PSD-95/SAP90 family.
Nehring RB, Wischmeyer E, Döring F, Veh RW, Sheng M, Karschin A. Nehring RB, et al. J Neurosci. 2000 Jan 1;20(1):156-62. doi: 10.1523/JNEUROSCI.20-01-00156.2000. J Neurosci. 2000. PMID: 10627592 Free PMC article. - Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs.
Craven SE, El-Husseini AE, Bredt DS. Craven SE, et al. Neuron. 1999 Mar;22(3):497-509. doi: 10.1016/s0896-6273(00)80705-9. Neuron. 1999. PMID: 10197530 - Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases.
Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Kim E, et al. Nature. 1995 Nov 2;378(6552):85-8. doi: 10.1038/378085a0. Nature. 1995. PMID: 7477295
Cited by
- A Specific Nutrient Combination Attenuates the Reduced Expression of PSD-95 in the Proximal Dendrites of Hippocampal Cell Body Layers in a Mouse Model of Phenylketonuria.
Bruinenberg VM, van Vliet D, Attali A, de Wilde MC, Kuhn M, van Spronsen FJ, van der Zee EA. Bruinenberg VM, et al. Nutrients. 2016 Mar 26;8(4):185. doi: 10.3390/nu8040185. Nutrients. 2016. PMID: 27102170 Free PMC article. - Palmitoylation of huntingtin by HIP14 is essential for its trafficking and function.
Yanai A, Huang K, Kang R, Singaraja RR, Arstikaitis P, Gan L, Orban PC, Mullard A, Cowan CM, Raymond LA, Drisdel RC, Green WN, Ravikumar B, Rubinsztein DC, El-Husseini A, Hayden MR. Yanai A, et al. Nat Neurosci. 2006 Jun;9(6):824-31. doi: 10.1038/nn1702. Epub 2006 May 14. Nat Neurosci. 2006. PMID: 16699508 Free PMC article. - Synaptic clustering of PSD-95 is regulated by c-Abl through tyrosine phosphorylation.
Perez de Arce K, Varela-Nallar L, Farias O, Cifuentes A, Bull P, Couch BA, Koleske AJ, Inestrosa NC, Alvarez AR. Perez de Arce K, et al. J Neurosci. 2010 Mar 10;30(10):3728-38. doi: 10.1523/JNEUROSCI.2024-09.2010. J Neurosci. 2010. PMID: 20220006 Free PMC article. - Differential palmitoylation directs the AMPA receptor-binding protein ABP to spines or to intracellular clusters.
DeSouza S, Fu J, States BA, Ziff EB. DeSouza S, et al. J Neurosci. 2002 May 1;22(9):3493-503. doi: 10.1523/JNEUROSCI.22-09-03493.2002. J Neurosci. 2002. PMID: 11978826 Free PMC article. - Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion.
Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. Mukai J, et al. Nat Neurosci. 2008 Nov;11(11):1302-10. doi: 10.1038/nn.2204. Epub 2008 Oct 5. Nat Neurosci. 2008. PMID: 18836441 Free PMC article.
References
- Aoki C., Bredt D.S., Fenstemaker S., Lubin M. The subcellular distribution of nitric oxide synthase relative to the NR1 subunit of NMDA receptors in the cerebral cortex. Prog. Brain Research. 1998;118:83–97. - PubMed
- Aoki C., Oviedo H., Alexandre L., Bredt D.S. Synaptic membranous and cytoplasmic localization of PSD-95EM-immunocytochemical detection with intact developing cortical neuropil Soc. Neurosci. Abstr. 510.8 1999. 1277a
- Arnold D.B., Clapham D.E. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron. 1999;23:149–157. - PubMed
- Brenman J.E., Chao D.S., Gee S.H., McGee A.W., Craven S.E., Santillano D.R., Huang F., Xia H., Peters M.F., Froehner S.C., Bredt D.S. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α-1 syntrophin mediated by PDZ motifs Cell 84 1996. 757 767a - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous