Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila - PubMed (original) (raw)
Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila
J Yoon et al. J Neurosci. 2000.
Abstract
The Drosophila trp gene encodes a light-activated Ca(2+) channel subunit, which is a prototypical member of a novel class of channel proteins. Previously identified trp mutants are all recessive, loss-of-function mutants characterized by a transient receptor potential and the total or near-total loss of functional TRP protein. Although retinal degeneration does occur in these mutants, it is relatively mild and slow in onset. We report herein a new mutant, Trp(P365), that does not display the transient receptor potential phenotype and is characterized by a substantial level of the TRP protein and rapid, semi-dominant degeneration of photoreceptors. We show that, in spite of its unusual phenotypes, Trp(P365) is a trp allele because a Trp(P365) transgene induces the mutant phenotype in a wild-type background, and a wild-type trp transgene in a Trp(P365) background suppresses the mutant phenotype. Moreover, amino acid alterations that could cause the Trp(P365) phenotype are found in the transmembrane segment region of the mutant channel protein. Whole-cell recordings clarified the mechanism underlying the retinal degeneration by showing that the TRP channels of Trp(P365) are constitutively active. Although several genes, when mutated, have been shown to cause retinal degeneration in Drosophila, the underlying mechanism has not been identified for any of them. The present studies provide evidence for a specific mechanism for massive degeneration of photoreceptors in Drosophila. Insofar as some human homologs of TRP are highly expressed in the brain, a similar mechanism could be a major contributor to degenerative disorders of the brain.
Figures
Fig. 1.
A, Comparison of the ERGs of_P365_-carrying mutants with those of wild type and_trp_. Representative ERGs recorded from wild type (a), P365/+ heterozygote (b), P365/P365_homozygote (c), null trp mutant,trpP343/trpP343(d), and flies carrying heteroallelic combinations of P365 with_trpP343 (e) and_trpCM_ (f). All flies were marked with the mutation w(white) to remove the red screening pigments in the eye. All recordings both in this figure and Figure 4 were obtained at 7 d after eclosion. For each recording shown, a white stimulus and an orange stimulus, each of 4 sec duration, were presented 20 sec apart, after 1 min of dark adaptation (bottom trace).B, Time course of disappearance of the deep pseudopupil as a measure of photoreceptor degeneration in P365/+ heterozygotes. The fraction of flies that have not lost the deep pseudopupils was determined in sample populations raised in 12 hr light/dark cycles (LD) or in complete darkness (DD) as a function of age in days after eclosion. All flies were marked with w (white).
Fig. 2.
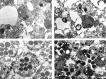
Electron micrographs of transverse sections of_P365_ mutant retinas near the R7 and R8 rhabdomere boundary. Retinas of P365 homozygotes raised in DD (A) and LD (B) at 0 d after eclosion. Retinas of P365/+ heterozygotes raised in DD (C) or LD (D) cycles at 2 d after eclosion. In D, some photoreceptors are missing, and some stain darkly (arrows). All flies were raised at 25°C. Rh1,.., Rh7: Rhabdomeres of R1,.., R7 photoreceptors. Scale bar, 2 μm.
Fig. 3.
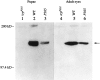
Western blot analysis of the P365_mutant and controls (wild-type and_trpP301) at late pupal and early adult stages. Lanes 1–3 were loaded with total pupal protein homogenates prepared from five pupae per lane harvested on the last day of pupal life, and _lanes 4–6_were loaded with total eye protein homogenates prepared from 10 eyes per lane dissected out within the first day after eclosion.Lanes 1, 4, trpP301;lanes 2, 5, wild type; lanes 3, 6, P365. The blots were labeled with a monoclonal anti-TRP antibody.
Fig. 4.
Localization of the TRP protein in_P365_ and wild-type rhabdomeres by immunofluorescence confocal microscopy. A, C, Confocal micrographs of the same group of wild-type rhabdomeres double-labeled with a TRP antiserum and phalloidin and visualized for TRP labeling (A) and phalloidin labeling (C), respectively. B, D, Confocal micrographs of the same group of P365 rhabdomeres double-labeled with a TRP antiserum and phalloidin and visualized for TRP labeling (B) and phalloidin labeling (D), respectively. All flies were marked with the mutation white to remove the screening pigments in the eye. The optical sections were <1-μm-thick near the distal tips of the rhabdomeres.
Fig. 5.
Induction of the P365 ERG phenotype in wild type using a P365 transgene and rescue of the_P365_ ERG phenotype using a_trp_+ transgene. A, Conversion of the wild-type phenotype (top) into a partial P365 phenotype by the introduction of one (middle) or two (bottom) copies of the P element transformation vector carrying the P365 allele of the trp gene P[P365_] into a wild-type background. B, Rescue of the heterozygous_P365/+ phenotype (top) using one (middle) or two (bottom) copies of the P element transformation vector carrying a wild-type allele of the_trp_ gene, P[trp+].C, Partial rescue of the homozygous_P365_/P365 phenotype (top) using one (middle) or two (bottom) copies of P[trp+]. Unlike most other flies used in this work, these flies had normal red eyes. The Carnegie 3 vector used for the generation of the P[trp+] transgene carries the_rosy_ (ry) gene as a marker, which is detected in a ry_− and_w+ background. Consequently, all experimental flies in B and C, carrying this vector, had normal red eyes. The pCaSpeR-3 vector used for the generation of the P[P365_] construct, on the other hand, carries a mini-white gene detected in a_w_− background. Experimental flies in this case had eye colors of varying shades of red. To remove a possible source of variations in the ERG size or shape, these flies were made fully red-eyed by introducing the w gene (A). All control flies in the top row also had normal red eyes. The age of flies and stimulus protocol were as in Figure 1_A.
Fig. 6.
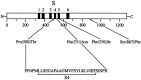
Deduced amino acid substitutions in the TRP protein of the TrpP365 mutant. The TRP protein is presented schematically at the top, with the transmembrane segments labeled S1 through S6 and amino acid positions labeled below (modified from Chevesich et al., 1997). The amino acid substitutions detected in_TrpP365_ are shown in the_middle_, with thin lines connecting the labels with the positions of the altered amino acids in the protein. The full amino acid sequence between the first two mutations is provided at the bottom. The fourth transmembrane segment sequence is labeled S4.
Fig. 7.
Single-cell functional analysis by whole-cell recordings. A shows a typical LIC of a wild-type cell (left trace) in response to an orange stimulus (OG 590 sharp-cut filter, 1 log unit neutral density filter) and the absence of any responses in_TrpP365_/trpCM_and_TrpP365/TrpP365(middle and right traces). The duration of the orange light stimulus is indicated above each trace.B–D compare families of current traces elicited by series of voltage steps from photoreceptors of wild type (left column), the light-insensitive cells of_TrpP365_/trpCM(middle column), and_TrpP365_/_TrpP365_homozygotes (right column). For each experiment, a series of nine voltage steps was applied from a holding potential of −20 mV in 20 mV steps (D, bottom traces).B, Membrane currents were recorded 30 sec after establishing the whole-cell configuration with physiological concentrations (1.5 m
m
) of Ca2+ in the bath. C, Application of 10 m
m
La3+ to the bath suppressed the membrane currents.D, Membrane currents obtained with 0 m
m
Ca2+ in the bath. In the case of wild type, the RDC is shown (left traces), which was allowed to develop by holding the cell in whole-cell configuration in a Ca2+-free medium for 12 min. In the mutant, the constitutive currents were recorded as soon as the whole-cell configuration was established. The currents shown were recorded 25 sec after establishing the whole-cell configuration. E, A histogram summarizing the data illustrated in Figure 7_B_from several different flies. The number of cells with constitutive activity at the time of establishing the whole-cell configuration is shown for each genotype.
Fig. 8.
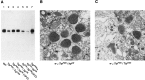
Western and EM analyses of_TrpP365_/trpCM_and controls. A, Western blot analyses of the heteroallelic mutant_TrpP365/trpCM(lanes 3, 4) and controls: wild type (lane 1),TrpP365/TrpP365_homozygotes (lane 2),TrpP365/+ heterozygotes (lane 7), and_trpCM/trpCM(lanes 5, 6). For_TrpP365_/trpCM_and_trpCM/trpCM, one of the two lanes contained samples prepared from flies raised at 19°C (so indicated). All other samples were from flies raised at 24°C. B, C, Electron micrographs of transverse sections through the ommatidial layer (at the level of R7 photoreceptor nuclei) of_TrpP365_/trpCM(B) and_TrpP365_/TrpP365(C), both raised at 19°C. All samples were obtained from newly eclosed adult flies, and all flies were marked with_w_. Scale bar, 1 μm. The_TrpP365_/TrpP365_mutant retina appears less degenerated in C above than in the sections shown in Figure 2, A and_B. The main reason is that the flies used for the above micrograph were raised at 19°C, whereas those used for Figure 2 were raised at 25°C. In addition, sections shown in Figure 2 were obtained from much more proximal levels of the retina than the one shown above, and, in fly eye, degeneration proceeds from the proximal to distal direction.
Similar articles
- Single amino acid change in the fifth transmembrane segment of the TRP Ca2+ channel causes massive degeneration of photoreceptors.
Hong YS, Park S, Geng C, Baek K, Bowman JD, Yoon J, Pak WL. Hong YS, et al. J Biol Chem. 2002 Sep 13;277(37):33884-9. doi: 10.1074/jbc.M204075200. Epub 2002 Jul 9. J Biol Chem. 2002. PMID: 12107168 - Complete RNAi rescue of neuronal degeneration in a constitutively active Drosophila TRP channel mutant.
Geng C, Pellegrino A, Bowman J, Zhu L, Pak WL. Geng C, et al. Biochim Biophys Acta. 2004 Sep 6;1674(1):91-7. doi: 10.1016/j.bbagen.2004.06.002. Biochim Biophys Acta. 2004. PMID: 15342118 - Exploring Excitotoxicity and Regulation of a Constitutively Active TRP Ca2+ Channel in Drosophila.
Shieh BH, Nuzum L, Kristaponyte I. Shieh BH, et al. Fly (Austin). 2021 Dec;15(1):8-27. doi: 10.1080/19336934.2020.1851586. Epub 2020 Dec 1. Fly (Austin). 2021. PMID: 33200658 Free PMC article. - The TRP calcium channel and retinal degeneration.
Minke B. Minke B. Adv Exp Med Biol. 2002;514:601-22. doi: 10.1007/978-1-4615-0121-3_34. Adv Exp Med Biol. 2002. PMID: 12596945 Review. - The roles of trp and calcium in regulating photoreceptor function in Drosophila.
Minke B, Selinger Z. Minke B, et al. Curr Opin Neurobiol. 1996 Aug;6(4):459-66. doi: 10.1016/s0959-4388(96)80050-x. Curr Opin Neurobiol. 1996. PMID: 8794093 Review.
Cited by
- Increase in membrane surface expression and phosphorylation of TRPC3 related to olfactory dysfunction in α-synuclein transgenic mice.
Chen M, Liu J, Luo H, Duan C, Gao G, Yang H. Chen M, et al. J Cell Mol Med. 2022 Oct;26(19):5008-5020. doi: 10.1111/jcmm.17524. Epub 2022 Aug 27. J Cell Mol Med. 2022. PMID: 36029194 Free PMC article. - Impaired Mitochondrial Energy Production Causes Light-Induced Photoreceptor Degeneration Independent of Oxidative Stress.
Jaiswal M, Haelterman NA, Sandoval H, Xiong B, Donti T, Kalsotra A, Yamamoto S, Cooper TA, Graham BH, Bellen HJ. Jaiswal M, et al. PLoS Biol. 2015 Jul 15;13(7):e1002197. doi: 10.1371/journal.pbio.1002197. eCollection 2015 Jul. PLoS Biol. 2015. PMID: 26176594 Free PMC article. - Phototransduction and retinal degeneration in Drosophila.
Wang T, Montell C. Wang T, et al. Pflugers Arch. 2007 Aug;454(5):821-47. doi: 10.1007/s00424-007-0251-1. Epub 2007 May 9. Pflugers Arch. 2007. PMID: 17487503 Review. - Human eye conditions: insights from the fly eye.
Gaspar P, Almudi I, Nunes MDS, McGregor AP. Gaspar P, et al. Hum Genet. 2019 Sep;138(8-9):973-991. doi: 10.1007/s00439-018-1948-2. Epub 2018 Nov 1. Hum Genet. 2019. PMID: 30386938 Review. - The role of TRP channels in oxidative stress-induced cell death.
Miller BA. Miller BA. J Membr Biol. 2006 Jan;209(1):31-41. doi: 10.1007/s00232-005-0839-3. Epub 2006 Apr 17. J Membr Biol. 2006. PMID: 16685599 Review.
References
- Armstrong CM, Hille B. Voltage-gated ion channels and electrical excitability. Neuron. 1998;20:371–380. - PubMed
- Arnon A, Cook B, Montell C, Selinger Z, Minke B. Calmodulin regulation of calcium stores in phototransduction of Drosophila. Science. 1997;275:1119–1121. - PubMed
- Baumann O, Walz B. Topography of Ca++-sequestering endoplasmic reticulum in photoreceptors and pigmented glial cells in the compound eye of the honeybee drone. Cell Tissue Res. 1989;255:511–522.
- Bentrop J. Rhodopsin mutations as the cause of retinal degenerations. Acta Anat. 1998;162:85–94. - PubMed
- Bloomquist BB, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and it role in phototransduction. Cell. 1988;54:723–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 EY000033/EY/NEI NIH HHS/United States
- R01 EY000033/EY/NEI NIH HHS/United States
- EY03529/EY/NEI NIH HHS/United States
- R37 EY000033/EY/NEI NIH HHS/United States
- R01 EY003529/EY/NEI NIH HHS/United States
- EY00033/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous