The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons - PubMed (original) (raw)
The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons
C Weiss et al. J Neurosci. 2000.
Abstract
The M1 muscarinic agonist CI-1017 was administered intravenously to aging rabbits on a daily basis before and during hippocampally dependent trace eyeblink conditioning sessions. Circulating levels of CI-1017 were significantly related to the drug dose. The drug was found to significantly increase the rate and amount of learning in a dose-dependent manner with no significant effects on the amplitude, area, or latency of conditioned responses. There was no evidence of pseudoconditioning at the highest drug concentration, and the minimally effective dose produced only mild and temporary hypersalivation as a side effect. CI-1017 (10 microM) was also found to increase the excitability of CA1 pyramidal neurons recorded from hippocampal slices from young and aging naive rabbits as measured by changes in spike-frequency adaptation and the postburst afterhyperpolarization. These biophysical changes were reversed with either atropine (1 microM) or pirenzepine (1 microM). These results suggest that M1 agonists ameliorate age-related learning and memory impairments at least in part by reducing the afterhyperpolarization and spike-frequency adaptation of hippocampal pyramidal neurons and that M1 agonists may be an effective therapy for reducing the cognitive deficits that accompany normal aging and/or Alzheimer's disease.
Figures
Fig. 1.
A, A graph of the percent of trials with CRs across 15 daily training sessions as a function of drug dose. B, The two higher doses (5 and 1 mg/ml) exhibited significantly more CRs than the two lower doses (0 and 0.5 mg/ml). Data are means ± SE. Error bars are omitted from A for clarity.
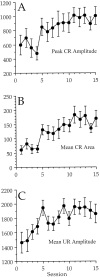
Fig. 2.
A, A graph of the peak amplitude of response during the CS–US period on trials in which there was a CR.B, A graph of the area of response during the CS–US interval on trials in which there was a CR. C, A graph of the mean amplitude of response during the UR period. There was a significant increase in all three measures across sessions, but no significant differences among the groups and no significant interaction of dose and session were found. A comparison of these data with those in Figure 1 indicate that the high-dose rabbits showed more CRs, rather than CRs with different characteristics. The _y_-axis is shown in integrated units.
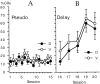
Fig. 3.
A, CI-1017 had no significant effect on the percent of trials with apparent CRs during 15 behavioral control sessions that presented unpaired tones and air puffs, i.e., there was no pseudoconditioning. B, CI-1017 had no significant effect on the rate of learning when the same rabbits were subsequently switched to the delay conditioning paradigm for five daily sessions. D, Drug at 5 mg/ml; V, vehicle control.
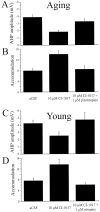
Fig. 4.
CI-1017 increased the excitability of both young and aging hippocampal pyramidal neurons by significantly reducing the amplitude of the AHP and spike-frequency adaptation. A, CI-1017 (10 μ
m
) significantly reduced the mean amplitude of the AHP in neurons from aging rabbits, and the effect was significantly reversed after application of the m1-specific antagonist pirenzepine (1 μ
m
). B, CI-1017 significantly reduced the spike-frequency adaptation of neurons from aging rabbits after application of the drug (10 μ
m
) to the bath; the effect was significantly reversed by the addition of pirenzepine (1 μ
m
). C, CI-1017 (10 μ
m
) reduced the mean amplitude of the AHP in neurons from young rabbits; the effect was reversed after application of the cholinergic antagonist atropine (1 μ
m
). D, CI-1017 (10 μ
m
) significantly reduced the spike-frequency adaptation of neurons from young rabbits; the effect was significantly reversed by the addition of atropine (1 μ
m
).
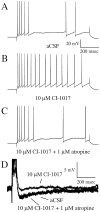
Fig. 5.
Typical examples of the effects of CI-1017 on biophysical properties from a single hippocampal CA1 pyramidal neuron from a young naive rabbit. An 800 msec pulse was used to examine accommodation after a burst of four action potentials.A, Accommodation of the neuron in aCSF.B, Accommodation is reduced by the addition of CI-1017, i.e., the cell is more excitable. C, The excitability change due to CI-1017 is reversed by the addition of the muscarinic antagonist atropine. D, Examples of the postburst AHP during control (aCSF), drug (CI-1017), and reversal (CI-1017 plus atropine) conditions.
Similar articles
- Cholinergic facilitation of trace eyeblink conditioning in aging rabbits.
Disterhoft JF, Kronforst-Collins M, Oh MM, Power JM, Preston AR, Weiss C. Disterhoft JF, et al. Life Sci. 1999;64(6-7):541-8. doi: 10.1016/s0024-3205(98)00599-2. Life Sci. 1999. PMID: 10069521 Review. - Modulation of cholinergic transmission enhances excitability of hippocampal pyramidal neurons and ameliorates learning impairments in aging animals.
Disterhoft JF, Matthew Oh M. Disterhoft JF, et al. Neurobiol Learn Mem. 2003 Nov;80(3):223-33. doi: 10.1016/j.nlm.2003.08.004. Neurobiol Learn Mem. 2003. PMID: 14521865 - Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation.
Thompson LT, Moyer JR Jr, Disterhoft JF. Thompson LT, et al. J Neurophysiol. 1996 Sep;76(3):1836-49. doi: 10.1152/jn.1996.76.3.1836. J Neurophysiol. 1996. PMID: 8890296 - Metrifonate increases neuronal excitability in CA1 pyramidal neurons from both young and aging rabbit hippocampus.
Oh MM, Power JM, Thompson LT, Moriearty PL, Disterhoft JF. Oh MM, et al. J Neurosci. 1999 Mar 1;19(5):1814-23. doi: 10.1523/JNEUROSCI.19-05-01814.1999. J Neurosci. 1999. PMID: 10024365 Free PMC article. - Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease.
Disterhoft JF, Oh MM. Disterhoft JF, et al. J Physiol Paris. 2006 Mar-May;99(2-3):180-92. doi: 10.1016/j.jphysparis.2005.12.079. Epub 2006 Feb 3. J Physiol Paris. 2006. PMID: 16458491 Review.
Cited by
- Galantamine facilitates acquisition of hippocampus-dependent trace eyeblink conditioning in aged rabbits.
Weible AP, Oh MM, Lee G, Disterhoft JF. Weible AP, et al. Learn Mem. 2004 Jan-Feb;11(1):108-15. doi: 10.1101/lm.69804. Learn Mem. 2004. PMID: 14747524 Free PMC article. - Cholinergic effects on fear conditioning II: nicotinic and muscarinic modulations of atropine-induced disruption of the degraded contingency effect.
Carnicella S, Pain L, Oberling P. Carnicella S, et al. Psychopharmacology (Berl). 2005 Apr;178(4):533-41. doi: 10.1007/s00213-004-2101-6. Epub 2005 Feb 5. Psychopharmacology (Berl). 2005. PMID: 15696332 - Long-lasting cholinergic modulation underlies rule learning in rats.
Saar D, Grossman Y, Barkai E. Saar D, et al. J Neurosci. 2001 Feb 15;21(4):1385-92. doi: 10.1523/JNEUROSCI.21-04-01385.2001. J Neurosci. 2001. PMID: 11160410 Free PMC article. - Acetylcholine release in the hippocampus and striatum during place and response training.
Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE. Pych JC, et al. Learn Mem. 2005 Nov-Dec;12(6):564-72. doi: 10.1101/lm.33105. Learn Mem. 2005. PMID: 16322358 Free PMC article. - Age-related deficits in neuronal physiology and cognitive function are recapitulated in young mice overexpressing the L-type calcium channel, CaV 1.3.
Moore SJ, Cazares VA, Temme SJ, Murphy GG. Moore SJ, et al. Aging Cell. 2023 Mar;22(3):e13781. doi: 10.1111/acel.13781. Epub 2023 Jan 26. Aging Cell. 2023. PMID: 36703244 Free PMC article.
References
- Akase E, Thompson LT, Disterhoft JF. A system for quantitative analysis of associative learning. II. Real-time software for MS-DOS microcomputers. J Neurosci Methods. 1994;54:119–130. - PubMed
- Bartus RT, Dean RL, Beer B, Lippa S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–416. - PubMed
- Benardo LS, Prince DA. Acetylcholine induced modulation of hippocampal pyramidal neurons. Brain Res. 1981;211:227–234. - PubMed
- Benardo LS, Prince DA. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982;249:315–331. - PubMed
- Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous