14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants - PubMed (original) (raw)
Comparative Study
14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants
T May et al. Plant Cell. 2000 Jan.
Abstract
Transit sequences of chloroplast-destined precursor proteins are phosphorylated on a serine or threonine residue. The amino acid motif around the phosphorylation site is related to the phosphopeptide binding motif for 14-3-3 proteins. Plant 14-3-3 proteins interact specifically with wheat germ lysate-synthesized chloroplast precursor proteins and require an intact phosphorylation motif within the transit sequence. Chloroplast precursor proteins do not interact with 14-3-3 when synthesized in the heterologous reticulocyte lysate. In contrast, a precursor protein destined for plant mitochondria was found to be associated with 14-3-3 proteins present in the reticulocyte lysate but not with 14-3-3 from wheat germ lysate. This indicates an unrecognized selectivity of 14-3-3 proteins for precursors from mitochondria and plastids in plants in comparison to fungi and animals. The heterooligomeric complex has an apparent size of 200 kD. In addition to the precursor protein, it contains 14-3-3 (probably as a dimer) and a heat shock protein Hsp70 isoform. Dissociation of the precursor complex requires ATP. Protein import experiments of precursor from the oligomeric complex into intact pea chloroplasts reveal three- to fourfold higher translocation rates compared with the free precursor, which is not complexed. We conclude that the 14-3-3-Hsp70-precursor protein complex is a bona fide intermediate in the in vivo protein import pathway in plants.
Figures
Figure 1.
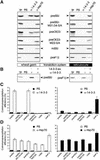
14-3-3 Proteins Interact with a Chloroplast Precursor–Containing Affinity Matrix. (A) Amino acid comparison between the phosphopeptide binding motif of 14-3-3 proteins and the phosphorylation site in the transit peptide (tp) of plastid precursor proteins (Waegemann and Soll, 1996; Yaffe et al., 1997). Letters in white indicate amino acids that are important for selectivity, letters in parentheses indicate those that are not essential, and letters in black indicate those that are variable. (B) PreSSU, mature SSU (mSSU), and ATPase subunit β precursor (preF1β) containing a C-terminal hexahistidine tag were bound to a Ni2+-chelating matrix. Wheat germ lysate was passed over the matrix, washed extensively, and eluted with 1 mM ATP followed by elution with a linear gradient of 50 to 500 mM NaCl. The last wash fraction (W), the ATP eluates (A), and the salt eluates (100 to 200 mM NaCl; black dots) were precipitated with trichloroacetic acid. Proteins were separated by SDS-PAGE followed by immunoblot analysis with antisera raised against Hsp70 and 14-3-3 proteins, as indicated by arrowheads. The immunoblot is shown.
Figure 2.
Anti–14-3-3 Antiserum Specifically Recognizes Wheat Germ 14-3-3 Proteins. Soluble proteins from a wheat germ lysate were separated by SDS-PAGE, transferred to nitrocellulose membranes, and incubated with an antiserum against a pea 14-3-3 protein either in the absence (−) or presence (+) of 2 μg of recombinant protein (14-3-3-ex).
Figure 3.
Chloroplast but Not Mitochondrial Precursor Proteins Interact with Plant 14-3-3 Proteins. The different proteins were translated in vitro in either the wheat germ or the reticulocyte lysate system. 35S-labeled translation products of the preSSU, preSSU–M31/34-S/A, mature SSU (mSSU), and preF1β as well as 3H-labeled translation products of preOE23 and preOE23–M22-S/A (as indicated by arrowheads) were coimmunoprecipitated by using antisera against 14-3-3 (α-14-3-3), Hsp70 (α-Hsp70), or preimmune serum (PIS). Ten percent of the labeled translation product (TP) was used as internal standard to calculate the immunoprecipitation efficiencies. Radioactive bands were cut out, dissolved in dimethyl sulfoxide, and quantified by liquid scintillation counting. (A) Typical autoradiograms. (B) Immunoprecipitations of the preSSU and preF1β translation product (as indicated by arrowheads) with antisera against 14-3-3 (α-14-3-3) were performed either in the absence (−) or presence (+) of 1 μg of recombinant pea 14-3-3 protein (14-3-3-ex). An autoradiogram is shown. (C) and (D) Quantification of immunoprecipitation efficiency for α-14-3-3 (C) and α-Hsp70 (D) antibodies, respectively. The calculations were conducted from a minimum of five or three independent experiments, respectively.
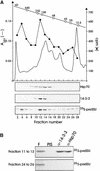
Figure 4.
PreSSU Forms a High Molecular Weight Complex in Wheat Germ Lysate. (A) A translation assay (50 μL volume) was fractionated by size exclusion chromatography calibrated with protein standards of different molecular mass (open circles). Thirty fractions of 500 μL were collected and precipitated with trichloroacetic acid, separated by SDS-PAGE, blotted, and analyzed by immunostaining with antisera raised to Hsp70 and to the 14-3-3 protein as well as by autoradiography (35S-preSSU). Radioactivity (filled diamonds) was quantified by liquid scintillation counting after dissolving the nitrocellulose in dimethyl sulfoxide. (B) Fractions 11 to 13 and 24 to 26 were combined, and the two pools were split into equal aliquots. Combined fractions were subjected to coimmunoprecipitation by antisera to 14-3-3 (α-14-3-3) and Hsp70 (α-Hsp70) as indicated. Ten percent of each combined fraction (F) was used as internal standard. An autoradiogram is shown. PIS, control experiment using preimmune serum.
Figure 5.
14-3-3 Forms a Heterooligomeric Complex with preSSU and Hsp70. (A) Wheat germ lysate–synthesized 35S-preSSU (filled diamonds) and the nonphosphorylatable mutant 35S-preSSU–M31/34-S/A (open diamonds) were fractionated in independent runs as before (see Figure 4) but with a fraction size of 250 μL. Only fractions of the relevant molecular size were collected. The positions of the molecular mass markers are indicated on top (open circles). Each fraction was precipitated by trichloroacetic acid, and precipitated proteins were separated by SDS-PAGE and subsequently transferred onto nitrocellulose membranes. Autoradiograms are shown below. Radioactivity present in each fraction was quantified by liquid scintillation counting after solubilizing nitrocellulose strips in dimethyl sulfoxide. (B) Wheat germ lysate (∼130 μg protein) was used for coimmunoprecipitation by anti–14-3-3 and anti-Hsp70 antibodies. The immunoprecipitated polypeptides were separated by SDS-PAGE, blotted, and immunodecorated using 14-3-3 and Hsp70 antisera, as indicated by arrowheads. PIS, control experiment using preimmune serum.
Figure 6.
Precursor-Containing Complex Dissociates in an ATP-Dependent Manner. (A) Freshly translated 35S-preSSU was fractionated by size exclusion chromatography (see Figure 4). Fractions 11 to 13 were combined, concentrated by ultrafiltration to a final volume of ∼150 μL, and divided into equal aliquots. Each aliquot was incubated for 15 min at 25°C in the absence (−ATP) or presence of different ATP concentrations as indicated. The incubation mixture was centrifuged at 165,000_g_ for 30 min. Proteins present in the supernatant (S) or pellet (I) were separated by SDS-PAGE, transferred onto nitrocellulose, and subjected to autoradiography. (B) Quantification of three independent results shown in (A). The sum of radioactivity in the soluble and the insoluble fractions, as determined by liquid scintillation counting, was taken as 100%. Values are given in percentages.
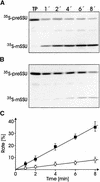
Figure 7.
Only Complexed but Not Free 35S-preSSU Is Imported into Isolated Chloroplasts with High Efficiency. (A) and (B) Freshly translated 35S-preSSU was fractionated by size exclusion chromatography (see Figure 4). Fractions 11 to 13 (A) and 24 to 26 (B) from three independent runs were combined and concentrated, and equal aliquots (100 μL) were used in each import reaction. Chloroplasts equivalent to 7.5 μg of chlorophyll were incubated with preSSU at 25°C for the different times indicated. The import reaction was stopped by adding ice-cold EDTA solution to a final concentration of 37.5 mM. Chloroplasts were recovered from the import reaction by centrifugation. Chloroplast proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes, and radioactivity recovered in processed mature SSU was determined by autoradiography. A typical autoradiogram out of a set of five independent experiments is shown. TP, 10% of translation product used as internal standard; 1′, 1 min. (C) Import efficiencies from complexed ([A], filled squares) or free ([B], open circles) preSSU as measured by the appearance of processed mature mSSU were quantified by liquid scintillation counting as above. The calculations were conducted from five independent experiments of five independent complex purifications. Values are given as percentages of the translation product supplied to each translocation reaction for the times indicated.
Similar articles
- Phosphorylation of the transit sequence of chloroplast precursor proteins.
Waegemann K, Soll J. Waegemann K, et al. J Biol Chem. 1996 Mar 15;271(11):6545-54. doi: 10.1074/jbc.271.11.6545. J Biol Chem. 1996. PMID: 8626459 - Investigations on the in vitro import ability of mitochondrial precursor proteins synthesized in wheat germ transcription-translation extract.
Dessi P, Pavlov PF, Wållberg F, Rudhe C, Brack S, Whelan J, Glaser E. Dessi P, et al. Plant Mol Biol. 2003 May;52(2):259-71. doi: 10.1023/a:1023993107220. Plant Mol Biol. 2003. PMID: 12856934 - Alternative processing of Arabidopsis Hsp70 precursors during protein import into chloroplasts.
Ratnayake RM, Inoue H, Nonami H, Akita M. Ratnayake RM, et al. Biosci Biotechnol Biochem. 2008 Nov;72(11):2926-35. doi: 10.1271/bbb.80408. Epub 2008 Nov 7. Biosci Biotechnol Biochem. 2008. PMID: 18997426 - Interaction of plant mitochondrial and chloroplast signal peptides with the Hsp70 molecular chaperone.
Zhang XP, Glaser E. Zhang XP, et al. Trends Plant Sci. 2002 Jan;7(1):14-21. doi: 10.1016/s1360-1385(01)02180-x. Trends Plant Sci. 2002. PMID: 11804822 Review. - Molecular chaperone involvement in chloroplast protein import.
Flores-Pérez Ú, Jarvis P. Flores-Pérez Ú, et al. Biochim Biophys Acta. 2013 Feb;1833(2):332-40. doi: 10.1016/j.bbamcr.2012.03.019. Epub 2012 Apr 12. Biochim Biophys Acta. 2013. PMID: 22521451 Review.
Cited by
- Targeting of an abundant cytosolic form of the protein import receptor at Toc159 to the outer chloroplast membrane.
Hiltbrunner A, Bauer J, Vidi PA, Infanger S, Weibel P, Hohwy M, Kessler F. Hiltbrunner A, et al. J Cell Biol. 2001 Jul 23;154(2):309-16. doi: 10.1083/jcb.200104022. J Cell Biol. 2001. PMID: 11470820 Free PMC article. - Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses.
NDong C, Danyluk J, Wilson KE, Pocock T, Huner NP, Sarhan F. NDong C, et al. Plant Physiol. 2002 Jul;129(3):1368-81. doi: 10.1104/pp.001925. Plant Physiol. 2002. PMID: 12114590 Free PMC article. - Identification of a signal that distinguishes between the chloroplast outer envelope membrane and the endomembrane system in vivo.
Lee YJ, Kim DH, Kim YW, Hwang I. Lee YJ, et al. Plant Cell. 2001 Oct;13(10):2175-90. doi: 10.1105/tpc.010232. Plant Cell. 2001. PMID: 11595795 Free PMC article. - The Principles of Protein Targeting and Transport Across Cell Membranes.
Chen Y, Shanmugam SK, Dalbey RE. Chen Y, et al. Protein J. 2019 Jun;38(3):236-248. doi: 10.1007/s10930-019-09847-2. Protein J. 2019. PMID: 31187382 Review. - Major Changes in Plastid Protein Import and the Origin of the Chloroplastida.
Knopp M, Garg SG, Handrich M, Gould SB. Knopp M, et al. iScience. 2020 Mar 27;23(3):100896. doi: 10.1016/j.isci.2020.100896. Epub 2020 Feb 8. iScience. 2020. PMID: 32088393 Free PMC article.
References
- Aitken, A. (1996). 14-3-3 and its possible role in coordinating multiple signaling pathways. Trends Cell Biol. 6 341–347. - PubMed
- Aitken, A., Collinge, D.B., van Heusden, B.P., Isobe, T., Roseboom, P.H., Rosenfeld, G., and Soll, J. (1991). 14-3-3 proteins: A highly conserved, widespread family of eukaryotic proteins. Trends Biochem. Sci. 17 498–501. - PubMed
- Anderson, C.W., Straus, J.W., and Dudock, B.S. (1983). Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 101 635–644. - PubMed
- Andrews, R.K., Harris, S.J., McNally, T., and Berndt, M.C. (1998). Binding of purified 14-3-3 ζ signaling protein to discrete amino acid sequences within the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX-V complex. Biochemistry 37 638–647. - PubMed
- Beckmann, R.P., Mizzen, L.E., and Welch, W.J. (1990). Interaction of Hsp70 with newly synthesized proteins: Implications for protein folding and assembly. Science 248 850–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources