The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell-cell contacts - PubMed (original) (raw)
The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell-cell contacts
D W Owens et al. Mol Biol Cell. 2000 Jan.
Free PMC article
Abstract
Despite the importance of epithelial cell contacts in determining cell behavior, we still lack a detailed understanding of the assembly and disassembly of intercellular contacts. Here we examined the role of the catalytic activity of the Src family kinases at epithelial cell contacts in vitro. Like E- and P-cadherin, Ca(2+) treatment of normal and tumor-derived human keratinocytes resulted in c-Yes (and c-Src and Fyn), as well as their putative substrate p120(CTN), being recruited to cell-cell contacts. A tyrosine kinase inhibitor with selectivity against the Src family kinases, PD162531, and a dominant-inhibitory c-Src protein that interferes with the catalytic function of the endogenous Src kinases induced cell-cell contact and E-cadherin redistribution, even in low Ca(2+), which does not normally support stable cell-cell adhesion. Time-lapse microscopy demonstrated that Src kinase inhibition induced stabilization of transiently formed intercellular contacts in low Ca(2+). Furthermore, a combination of E- and P-cadherin-specific antibodies suppressed cell-cell contact, indicating cadherin involvement. As a consequence of contact stabilization, normal cells were unable to dissociate from an epithelial sheet formed at high density and repair a wound in vitro, although individual cells were still motile. Thus, cadherin-dependent contacts can be stabilized both by high Ca(2+) and by inhibiting Src activity in low (0.03 mM) Ca(2+) in vitro.
Figures
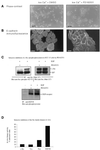
Figure 1
c-Yes and p120CTN translocate to cell–cell adhesions in epithelial cells in response to high Ca2+. Shown are confocal immunofluorescence micrographs of primary human epidermal keratinocytes, HEKs (A–E), and SCC-13 (F and G) cells stained with anti-c-Yes (A, B, C, F, and G) or anti-p120CTN (D and E). HEKs were maintained in low Ca2+ (A and D) or transferred to high Ca2+ for 24 h (B, C, and E). SCC-13 cells were transferred to low-Ca2+–containing medium for 24 h (F) and were subsequently transferred to high Ca2+ for 24 h (G). Cytochalasin D was added to HEKs at a concentration of 5 μmg/ml as they were transferred to high Ca2+ (C). Bars, 25 μm.
Figure 2
The Src-selective inhibitor PD162531 induces cell–cell contact and E-cadherin redistribution. (A) Phase-contrast images; (B) E-cadherin confocal immunofluorescence micrographs of HEKs maintained in low Ca2+ and treated with 0.02% DMSO (vol/vol) as controls (left panels) or with 2 μM PD162531 for 24 h (right panels). Bars, (A) 200 μm; (B) 25 μm. (C) SCC-13 tumor-derived keratinocytes, which also responded to PD162531, were grown in KGM serum-free medium and were deprived of exogenous EGF for 16 h, before restimulation with 2 ng/ml EGF (+ EGF) for 10 min in the absence (−) or presence (+) of 2 μM PD162531. Presumed c-Src autophosphorylation was monitored by immunoprecipitating cell lysates with anti-Src (mAb 327) and immunoblotting using anti-Src (phospho-423-Y) as probe (upper left panel). Control immunoprecipitations were blotted with anti-Src (mAb 327; upper right panel). EGF-R autophosphorylation was monitored by immunoprecipitating untreated (− EGF) or EGF-treated (+ EGF) cell lysates with anti-EGF-R and immunoblotting with anti-phosphotyrosine. (D) In vitro kinase activities were measured as described in MATERIALS AND METHODS in the presence and absence of 100 nM PD162531. The kinase activities in the presence of drug are expressed as a percentage of untreated activities.
Figure 3
Induction of a kinase-defective (KD) c-Src protein also induces cell–cell contact and E-cadherin redistribution. (A) Immunoblot using avian-specific mAb EC10 to detect only exogenous c-Src-KD expression in SCC 13 parental cells and SCC-13/ecSrc-KD transfectant cells that were untreated (uninduced), treated with ethanol alone (control), or treated with 10 μM muristerone A for 24 h (upper panel). Both endogenous and exogneous Src expression were monitored by immunoblotting with anti-Src (mAb 327) that reacts with both human and avian Src (lower panel), (B) Phase-contrast images; (C) E-cadherin confocal immunofluorescence micrographs of SCC-13/ecSrc-KD cells maintained in low Ca2+ and untreated (left panels) or treated with 10 μM muristerone A for 24 h (right panels). Bars, (B) 200 μm; (C) 25 μm.
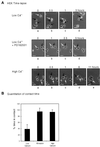
Figure 4
The Src-inhibitory drug PD162531 stabilizes cell–cell contacts. (A) Phase-contrast time-lapse images of low-density HEKs (in low Ca2+), which were treated with 0.02% DMSO (control, upper panel) or 2 μM PD162531 (middle panel) or transferred to high-Ca2+ medium (lower panel). Treatment with PD162531 or high Ca2+ began at 0 h and was continuous for the duration of the experiment. Individual cells are indicated by arrows. Bars, 50 μm. (B) The mean time that individual keratinocytes grown under different conditions spent in contact with other cells in the culture was determined by observing time-lapse images frame by frame. The duration of each intercellular contact formed by every cell in the time-lapse field (typically ∼20 cells per experiment) was recorded and expressed as a percentage of the experiment time (6 h), and the mean contact time was plotted as percentage of time in contact. Thus, a value of 100% would indicate that all cell–cell contacts persisted for the duration of the experiment. (C) The migration of individual cells that did not come into contact with other cells (typically approximately six cells per experiment) was quantitated using Openlab software, and the mean pixels per frame value for control cells in low Ca2+ was defined as 100%. (D) c-Yes confocal immunofluorescence micrograph of HEKs maintained in low Ca2+. Specifically shown here is the contact region between two cells that have collided. Bars, 25 μm.
Figure 4
The Src-inhibitory drug PD162531 stabilizes cell–cell contacts. (A) Phase-contrast time-lapse images of low-density HEKs (in low Ca2+), which were treated with 0.02% DMSO (control, upper panel) or 2 μM PD162531 (middle panel) or transferred to high-Ca2+ medium (lower panel). Treatment with PD162531 or high Ca2+ began at 0 h and was continuous for the duration of the experiment. Individual cells are indicated by arrows. Bars, 50 μm. (B) The mean time that individual keratinocytes grown under different conditions spent in contact with other cells in the culture was determined by observing time-lapse images frame by frame. The duration of each intercellular contact formed by every cell in the time-lapse field (typically ∼20 cells per experiment) was recorded and expressed as a percentage of the experiment time (6 h), and the mean contact time was plotted as percentage of time in contact. Thus, a value of 100% would indicate that all cell–cell contacts persisted for the duration of the experiment. (C) The migration of individual cells that did not come into contact with other cells (typically approximately six cells per experiment) was quantitated using Openlab software, and the mean pixels per frame value for control cells in low Ca2+ was defined as 100%. (D) c-Yes confocal immunofluorescence micrograph of HEKs maintained in low Ca2+. Specifically shown here is the contact region between two cells that have collided. Bars, 25 μm.
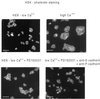
Figure 5
The contacts stabilized as a result of Src kinase inhibition are cadherin dependent. Immunofluorescence images of cells stained with phalloidin-FITC are shown for HEKs in low Ca2+ (top left), high Ca2+ (top right), and low Ca2+ in the presence of the Src-inhibitory drug PD162531 (bottom panels) in the absence (left panel) and presence of a mixture of E- and P-cadherin-specific antibodies (right panel). Bars 100 μm.
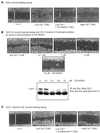
Figure 6
The Src-inhibitory drug PD162531 and overexpression of the dominant-negative c-Src protein suppress keratinocyte wound healing in vitro. Phase-contrast photomicrographs show four individual HEK monolayers (A) or SCC-13/ecSrc-KD cells (B and C) grown to confluence in low Ca2+ and then wounded (example shown at t = 0) and either treated for 16 h (16h) with 0.02% DMSO (low Ca2+, 0 μM), 0.1, 1, 2, or 10 μM PD162531 as indicated (low Ca2+ + PD162531), or 10 μM muristerone A [low Ca2+ muristerone A (16h)] or transferred to high Ca2+ for 16 h [high Ca2+ (16h)]. Bars, 200 μm. White bars represent relative distances between the two edges of apposing epithelial sheets. Presumed c-Src autophosphorylation at the indicated drug concentrations was monitored by immunoprecipitating cell lysates with anti-Src (mAb 327) and immunoblotting using anti-Src (phospho-423-Y) as probe (B).
Figure 7
Model depicting the likely biological consequences of modulating Src kinase activity in E-cadherin-expressing epithelial cells. The weakening of intercellular contact stability may facilitate processes such as epithelial wound repair or cancer cell invasion.
Similar articles
- Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion.
Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Paolo Dotto G. Calautti E, et al. J Cell Biol. 1998 Jun 15;141(6):1449-65. doi: 10.1083/jcb.141.6.1449. J Cell Biol. 1998. PMID: 9628900 Free PMC article. - pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions.
Volberg T, Romer L, Zamir E, Geiger B. Volberg T, et al. J Cell Sci. 2001 Jun;114(Pt 12):2279-89. doi: 10.1242/jcs.114.12.2279. J Cell Sci. 2001. PMID: 11493667 - Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion.
Calautti E, Grossi M, Mammucari C, Aoyama Y, Pirro M, Ono Y, Li J, Dotto GP. Calautti E, et al. J Cell Biol. 2002 Jan 7;156(1):137-48. doi: 10.1083/jcb.200105140. Epub 2002 Jan 3. J Cell Biol. 2002. PMID: 11777936 Free PMC article. - The SRC-induced mesenchymal state in late-stage colon cancer cells.
Avizienyte E, Brunton VG, Fincham VJ, Frame MC. Avizienyte E, et al. Cells Tissues Organs. 2005;179(1-2):73-80. doi: 10.1159/000084511. Cells Tissues Organs. 2005. PMID: 15942195 Review. - The p60c-src family of protein-tyrosine kinases: structure, regulation, and function.
Brickell PM. Brickell PM. Crit Rev Oncog. 1992;3(4):401-46. Crit Rev Oncog. 1992. PMID: 1384720 Review.
Cited by
- The effects of c-Src kinase on EMT signaling pathway in human lens epithelial cells associated with lens diseases.
Li X, Wang F, Ren M, Du M, Zhou J. Li X, et al. BMC Ophthalmol. 2019 Nov 8;19(1):219. doi: 10.1186/s12886-019-1229-4. BMC Ophthalmol. 2019. PMID: 31703690 Free PMC article. - Regulation of adherens junction dynamics by phosphorylation switches.
Bertocchi C, Vaman Rao M, Zaidel-Bar R. Bertocchi C, et al. J Signal Transduct. 2012;2012:125295. doi: 10.1155/2012/125295. Epub 2012 Jul 12. J Signal Transduct. 2012. PMID: 22848810 Free PMC article. - E-cadherin and Src associate with extradesmosomal Dsg3 and modulate desmosome assembly and adhesion.
Rötzer V, Hartlieb E, Vielmuth F, Gliem M, Spindler V, Waschke J. Rötzer V, et al. Cell Mol Life Sci. 2015 Dec;72(24):4885-97. doi: 10.1007/s00018-015-1977-0. Epub 2015 Jun 27. Cell Mol Life Sci. 2015. PMID: 26115704 Free PMC article. - Elevated Src family kinase activity stabilizes E-cadherin-based junctions and collective movement of head and neck squamous cell carcinomas.
Veracini L, Grall D, Schaub S, Beghelli-de la Forest Divonne S, Etienne-Grimaldi MC, Milano G, Bozec A, Babin E, Sudaka A, Thariat J, Van Obberghen-Schilling E. Veracini L, et al. Oncotarget. 2015 Apr 10;6(10):7570-83. doi: 10.18632/oncotarget.3071. Oncotarget. 2015. PMID: 25779657 Free PMC article. - Activation of Smad-mediated TGF-β signaling triggers epithelial-mesenchymal transitions in murine cloned corneal progenitor cells.
Kawakita T, Espana EM, Higa K, Kato N, Li W, Tseng SC. Kawakita T, et al. J Cell Physiol. 2013 Jan;228(1):225-34. doi: 10.1002/jcp.24126. J Cell Physiol. 2013. PMID: 22674610 Free PMC article.
References
- Behrens J, Vaket L, Friis R, Winterhager E, Van Roy F, Mareel M, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. - PMC - PubMed
- Birchmeier W, Behrens J. Cadherin expresssion in carcinomas; role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. - PubMed
- Birchmeier W, Weidner KM, Hulsken J, Behrens J. Molecular mechanisms leading to cell junction (cadherin) deficiency in invasive carcinomas. Semin Cancer Biol. 1993;4:231–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous