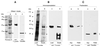Toxofilin, a novel actin-binding protein from Toxoplasma gondii, sequesters actin monomers and caps actin filaments - PubMed (original) (raw)
Toxofilin, a novel actin-binding protein from Toxoplasma gondii, sequesters actin monomers and caps actin filaments
O Poupel et al. Mol Biol Cell. 2000 Jan.
Free PMC article
Abstract
Toxoplasma gondii relies on its actin cytoskeleton to glide and enter its host cell. However, T. gondii tachyzoites are known to display a strikingly low amount of actin filaments, which suggests that sequestration of actin monomers could play a key role in parasite actin dynamics. We isolated a 27-kDa tachyzoite protein on the basis of its ability to bind muscle G-actin and demonstrated that it interacts with parasite G-actin. Cloning and sequence analysis of the gene coding for this protein, which we named Toxofilin, showed that it is a novel actin-binding protein. In in vitro assays, Toxofilin not only bound to G-actin and inhibited actin polymerization as an actin-sequestering protein but also slowed down F-actin disassembly through a filament end capping activity. In addition, when green fluorescent protein-tagged Toxofilin was overexpressed in mammalian nonmuscle cells, the dynamics of actin stress fibers was drastically impaired, whereas green fluorescent protein-Toxofilin copurified with G-actin. Finally, in motile parasites, during gliding or host cell entry, Toxofilin was localized in the entire cytoplasm, including the rear end of the parasite, whereas in intracellular tachyzoites, especially before they exit from the parasitophorous vacuole of their host cell, Toxofilin was found to be restricted to the apical end.
Figures
Figure 1
The T. gondii 27-kDa protein binds to G-actin. (A) G-actin chromatography. A 100,000 × g T. gondii cytosolic extract was subjected to affinity chromatography over a rabbit muscle G-actin column. Proteins bound to G-actin were eluted by 3 mM MgCl2 and 1 mM ATP. These proteins were further separated by SDS-PAGE on a 12% gel, transferred to a nitrocellulose membrane, and visualized with red Ponceau S stain. Lane b, cytosolic fraction corresponding to 5 × 107 T. gondii tachyzoites; lane a, eluate. The polypeptide migrating with a molecular mass of ∼27 kDa is marked with a triangle. (B) Native gel electrophoresis. A 100,000 × g parasite cytosolic extract (T.g) was incubated with IAEDANS-labeled G-actin (G), and the protein complexes were separated by native gel electrophoresis. The bands were visualized under a UV lamp (λ = 312 nm). Lane a, three bands were detected and marked, respectively, *0, *1, and *2; lane b, parasite cytosolic extract without fluorescent actin; lane c, fluorescent G-actin; lane d, fluorescent G-actin incubated under polymerization conditions to reach steady state before native gel electrophoresis. (C) Ponceau S staining on blot. Slices of the native gel (see B) containing either complex *0, *1, or *2 or from the adjacent lanes from positions corresponding to the migration of *0, *1, or *2 were boiled in SDS-PAGE sample buffer and analyzed on a 12% gel. The separated proteins were subsequently transferred onto a nitrocellulose membrane and stained with Ponceau S. Lanes a and b, material from bands *1 and *2, respectively. Apart from actin, three other similar bands were detectable in the complexes *1 and *2, including a band of ∼27 kDa (triangle); lane c, sample extracted from a slice cut from lane b on the native gel. The slice corresponded to polypeptides comigrating with *1; lane d, material from the band *0 from the native gel; lane e, material containing G-actin from lane c of the native gel.
Figure 2
Recombinant Toxofilin controls actin dynamics in vitro: it binds directly to actin monomers and caps actin filaments. (A) Native gel electrophoresis. IAEDANS-labeled G-actin was incubated with or without r-Toxofilin (lanes b and a, respectively) before electrophoresis on a native gel. (B) Pyrene-labeled G-actin polymerization assay. G-actin at 3 μM and 1% pyrene labeled was incubated with or without r-Toxofilin before induction of polymerization. The kinetics of actin polymerization was monitored in a Spex fluorimeter (F-2000; Hitachi) at λex = 350 nm and λem = 390 nm. +, kinetics of actin polymerization in the absence of r-Toxofilin; ∗, with 1 μM r-Toxofilin; ○, with 2 μM r-Toxofilin. Fluorescence intensity is measured in arbitrary units. Steady-state values are given in parentheses. Inset, upper panel, the amount of G and F-actin pelleted after ultracentrifugation (2 h, 100,000 × g, 4°C; as described by Tardieux et al., 1998); lower panel, amount of r-Toxofilin recovered in the supernatant (G) and pellet (F). (C) Pyrene-labeled F-actin depolymerization assay. Ten micromolar steady-state F-actin was induced to spontaneously depolymerize upon dilution to 2 μM. The kinetics of actin depolymerization was illustrated by the curve labeled +. Kinetics of actin depolymerization was also followed in the presence of r-Toxofilin at 1.5 μM (∗) and 2 μM (○). Inset, upper panel, amount of G and F-actin; lower panel, amount of r-Toxofilin recovered in the supernatant (G) and pellet (F).
Figure 3
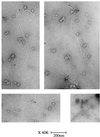
Toxofilin localizes at one end of actin filaments. Actin filaments at steady state (2 μM) were incubated with purified r-Toxofilin (1 μM). Immunolocalization of r-Toxofilin was revealed by anti-rabbit antibodies conjugated to 5-nm gold (see arrows). Actin filaments were negatively stained with 2% uranyl acetate.
Figure 4
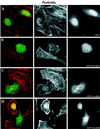
Expression of GFP-Toxofilin fusion protein affects actin dynamics in mammalian nonmuscle cells. HeLa cells (50% confluent) were transfected by the CaCl2 phosphate method with plamids encoding either GFP-Toxofilin or GFP (see MATERIALS AND METHODS) and were incubated overnight before replating on glass coverslips. The cells were incubated for 20 h (37°C, 5% CO2) before processing for immunofluorescence (see MATERIALS AND METHODS). For revealing the F-actin or Toxofilin, the cells were permeabilized after fixation and stained with either rhodamine-phalloidin or with an anti-Toxofilin antibody, respectively. (A) Phalloidin staining of F-actin in HeLa cells expressing GFP. Well-organized actin stress fibers are visible. (B–D) Phalloidin staining of F-actin in cells expressing GFP-Toxofilin. Actin stress fibers are disorganized. (D) Different levels of expression of the GFP-Toxofilin. (E) Detection of Toxofilin with an anti-Toxofilin antibody in HeLa cells transfected with the GFP-Toxofilin-encoding plasmid.
Figure 4
Expression of GFP-Toxofilin fusion protein affects actin dynamics in mammalian nonmuscle cells. HeLa cells (50% confluent) were transfected by the CaCl2 phosphate method with plamids encoding either GFP-Toxofilin or GFP (see MATERIALS AND METHODS) and were incubated overnight before replating on glass coverslips. The cells were incubated for 20 h (37°C, 5% CO2) before processing for immunofluorescence (see MATERIALS AND METHODS). For revealing the F-actin or Toxofilin, the cells were permeabilized after fixation and stained with either rhodamine-phalloidin or with an anti-Toxofilin antibody, respectively. (A) Phalloidin staining of F-actin in HeLa cells expressing GFP. Well-organized actin stress fibers are visible. (B–D) Phalloidin staining of F-actin in cells expressing GFP-Toxofilin. Actin stress fibers are disorganized. (D) Different levels of expression of the GFP-Toxofilin. (E) Detection of Toxofilin with an anti-Toxofilin antibody in HeLa cells transfected with the GFP-Toxofilin-encoding plasmid.
Figure 5
T. gondii Toxofilin binds to parasite G-actin and is associated with a parasite fraction containing F-actin. (A) DNase 1 affinity chromatography. DNase 1-bound proteins were eluted in SDS-PAGE sample buffer and separated on a 12% acrylamide gel. After transfer to a nitrocellulose membrane, eluted proteins were visualized with Ponceau S staining (lane a). Lane b, the eluate was probed for actin with an anti-actin antibody specific for T. gondii actin; lane c, the eluate was probed for Toxofilin with an anti-Toxofilin antibody. Toxofilin is marked with a star. (B) Toxofilin immunoprecipitation. A 100,000 × g parasite cytosolic extract was prepared from [35S]methionine-cysteine-labeled tachyzoites (lane a, load) and immunoprecipitated with anti-Toxofilin antibody (lane b, eluate). Toxofilin is marked with a star. Both the cytosolic extract and the immunoprecipitate contained actin (○) as assessed by Western blot using an anti-T. gondii actin-specific antibody (lanes c and d, respectively). (C) Protein fractionation. Parasite proteins were extracted in F-actin-stabilizing conditions to recover fractions containing G-actin (lane a) or F-actin (lane b; ○. Both fractions contained Toxofilin (∗) as assessed by Western blot analysis using an anti-Toxofilin antibody (lanes c and d, respectively).
Figure 6
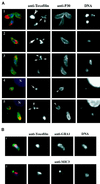
Toxofilin localization during tachyzoite motility and host cell entry is dynamic. (A, 1–3) Tachyzoites were allowed to glide on glass slides before being fixed and stained for the major surface protein P30 with mouse monoclonal anti-P30 followed by a secondary anti-mouse antibody conjugated to Alexa-488. Cells were then permeabilized and stained for Toxofilin using the anti-Toxofilin antibody followed by an anti-rabbit antibody conjugated to Alexa-568. Nuclei were stained by DAPI (see MATERIALS AND METHODS). In green, the surface of the parasite; in red, Toxofilin distribution; in blue, nuclei. (A, 4 and 5) Tachyzoites were incubated for 10 min with HFF cells plated on glass coverslips. Coverslips were washed in PBS and processed for immunofluorescence (see MATERIALS AND METHODS) either immediately or after further incubation for 10 or 20 h (37°C, 5% CO2). Extracellular tachyzoites were stained with the anti-P30 followed by the anti-mouse antibody conjugated to Alexa-488. The cells were subsequenty permeabilized and stained for Toxofilin followed by an anti-rabbit antibody conjugated to Alexa 568. Nuclei were stained by DAPI. Extracellular parasites were detected by the green fluorescent staining on their surface, whereas tachyzoites in the process of entering the host cell present an incomplete staining of their surface (arrows). (B) Gliding tachyzoites were processed as for A. After fixation they were permeabilized and stained for either their micronemes, using the mouse monoclonal antibody anti-MIC1, or for their dense granules, using the mouse monoclonal antibody anti-GRA3. In both cases, the secondary anti-mouse antibody was conjugated to Alexa-488, and the staining is shown in green. After removal of the unbound conjugated antibody, the cells were stained for Toxofilin and for DNA as described above.
Figure 7
In intracellular tachyzoites, Toxofilin distributes uniformly in the apical cytoplasm. Tachyzoites were incubated for 10 min (37°C, 5% CO2) with HFF cells plated on glass coverslips. The nonbound parasites were removed by changing the medium, and the infected cells were further incubated for 20 h as described in MATERIALS AND METHODS. At the end of the incubation period the cells were fixed, and extracellular tachyzoites were stained with the anti-P30 antibody followed by the anti-mouse antibody conjugated to Alexa-488, seen in red. The cells were subsequently permeabilized and stained for Toxofilin followed by an anti-rabbit conjugated to Alexa-568. Nuclei were stained by DAPI, shown here in green (artificial color). The actin cytoskeleton of the host cell was stained with rhodamine-phalloidin, shown in blue (artificial color). The indicated area was enlarged 2× and is presented as an inset.
Similar articles
- The toxofilin-actin-PP2C complex of Toxoplasma: identification of interacting domains.
Jan G, Delorme V, David V, Revenu C, Rebollo A, Cayla X, Tardieux I. Jan G, et al. Biochem J. 2007 Feb 1;401(3):711-9. doi: 10.1042/BJ20061324. Biochem J. 2007. PMID: 17014426 Free PMC article. - Actin dynamics is controlled by a casein kinase II and phosphatase 2C interplay on Toxoplasma gondii Toxofilin.
Delorme V, Cayla X, Faure G, Garcia A, Tardieux I. Delorme V, et al. Mol Biol Cell. 2003 May;14(5):1900-12. doi: 10.1091/mbc.e02-08-0462. Epub 2003 Feb 6. Mol Biol Cell. 2003. PMID: 12802063 Free PMC article. - Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion.
Delorme-Walker V, Abrivard M, Lagal V, Anderson K, Perazzi A, Gonzalez V, Page C, Chauvet J, Ochoa W, Volkmann N, Hanein D, Tardieux I. Delorme-Walker V, et al. J Cell Sci. 2012 Sep 15;125(Pt 18):4333-42. doi: 10.1242/jcs.103648. Epub 2012 May 28. J Cell Sci. 2012. PMID: 22641695 Free PMC article. - Toxoplasma gondii: perfecting an intracellular life style.
Sibley LD. Sibley LD. Traffic. 2003 Sep;4(9):581-6. doi: 10.1034/j.1600-0854.2003.00117.x. Traffic. 2003. PMID: 12911812 Review. - Where is the EXIT? Phenotypic screens for new egress factors in apicomplexan parasites.
Jimenéz-Ruiz E, Li W, Meissner M. Jimenéz-Ruiz E, et al. Mol Microbiol. 2024 Mar;121(3):359-367. doi: 10.1111/mmi.15166. Epub 2023 Sep 22. Mol Microbiol. 2024. PMID: 37740453 Review.
Cited by
- The toxofilin-actin-PP2C complex of Toxoplasma: identification of interacting domains.
Jan G, Delorme V, David V, Revenu C, Rebollo A, Cayla X, Tardieux I. Jan G, et al. Biochem J. 2007 Feb 1;401(3):711-9. doi: 10.1042/BJ20061324. Biochem J. 2007. PMID: 17014426 Free PMC article. - Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles.
Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. Alexander DL, et al. PLoS Pathog. 2005 Oct;1(2):e17. doi: 10.1371/journal.ppat.0010017. Epub 2005 Oct 21. PLoS Pathog. 2005. PMID: 16244709 Free PMC article. - The actin multigene family of Paramecium tetraurelia.
Sehring IM, Mansfeld J, Reiner C, Wagner E, Plattner H, Kissmehl R. Sehring IM, et al. BMC Genomics. 2007 Mar 28;8:82. doi: 10.1186/1471-2164-8-82. BMC Genomics. 2007. PMID: 17391512 Free PMC article. - ARL4D recruits cytohesin-2/ARNO to modulate actin remodeling.
Li CC, Chiang TC, Wu TS, Pacheco-Rodriguez G, Moss J, Lee FJ. Li CC, et al. Mol Biol Cell. 2007 Nov;18(11):4420-37. doi: 10.1091/mbc.e07-02-0149. Epub 2007 Sep 5. Mol Biol Cell. 2007. PMID: 17804820 Free PMC article. - Characterization of a differentially expressed protein that shows an unusual localization to intracellular membranes in Leishmania major.
Knuepfer E, Stierhof YD, McKean PG, Smith DF. Knuepfer E, et al. Biochem J. 2001 Jun 1;356(Pt 2):335-44. doi: 10.1042/0264-6021:3560335. Biochem J. 2001. PMID: 11368759 Free PMC article.
References
- Ajioka JW, et al. Gene discovery by EST sequencing in Toxoplasma gondii reveals sequences restricted to the Apicomplexa. PCR Methods Appl. 1998;8:18–28. - PubMed
- Allen ML, Dobrowolski JM, Muller H, Sibley LD, Mansour TE. Cloning and characterization of actin depolymerizing factor from Toxoplasma gondii. Mol Biochem Parasitol. 1997;88:43–52. - PubMed
- Ayscough KR. In vivo functions of actin-binding proteins. Curr Opin Cell Biol. 1998;10:102–111. - PubMed
- Boothroyd JC, Black M, Kim K, Pfefferkorn ER, Seeber F, Sibley LD, Soldati D. Forward and reverse genetics in the study of the obligate, intracellular parasite Toxoplasma gondii. In: Adolph KW, editor. Methods in Molecular Genetics. Vol. 6. San Diego: Academic Press; 1995. pp. 3–29.
- Carlier MF. Actin polymerization and ATP hydrolysis. Adv Biophys. 1990;26:51–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous