Specific chemical and structural damage to proteins produced by synchrotron radiation - PubMed (original) (raw)
Comparative Study
Specific chemical and structural damage to proteins produced by synchrotron radiation
M Weik et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Radiation damage is an inherent problem in x-ray crystallography. It usually is presumed to be nonspecific and manifested as a gradual decay in the overall quality of data obtained for a given crystal as data collection proceeds. Based on third-generation synchrotron x-ray data, collected at cryogenic temperatures, we show for the enzymes Torpedo californica acetylcholinesterase and hen egg white lysozyme that synchrotron radiation also can cause highly specific damage. Disulfide bridges break, and carboxyl groups of acidic residues lose their definition. Highly exposed carboxyls, and those in the active site of both enzymes, appear particularly susceptible. The catalytic triad residue, His-440, in acetylcholinesterase, also appears to be much more sensitive to radiation damage than other histidine residues. Our findings have direct practical implications for routine x-ray data collection at high-energy synchrotron sources. Furthermore, they provide a direct approach for studying the radiation chemistry of proteins and nucleic acids at a detailed, structural level and also may yield information concerning putative "weak links" in a given biological macromolecule, which may be of structural and functional significance.
Figures
Figure 1
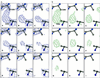
Sequential Fourier maps showing the time course of cleavage of the Cys-254–Cys-265 disulfide bond in _Tc_AChE. Cysteine residues were refined as alanine residues to avoid model bias. (a) 3Fo-2Fc maps, contoured at 1.5 σ. (b) Fo-Fc maps, contoured at 3 σ.
Figure 2
Sequential Fourier maps showing the time course of structural changes in the Cys-402–Cys-521 disulfide bond in _Tc_AChE. Data collection and refinement were as in Fig. 1. (a) 3Fo-2Fc maps, contoured at 1.5 σ. (b) Fo-Fc maps, contoured at 3 σ.
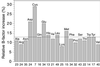
Figure 3
Histogram showing the increase in B factors for the side chains of the different types of amino acid in _Tc_AChE as a consequence of synchrotron irradiation. The horizontal line indicates the mean increase in side-chain B factors. The numbers along the _x_-axis show the number of occurrences of each type of amino acid in _Tc_AChE. The individual bars show the average increase in B factor for each type of amino acid for the second data set (B), as compared with the first data set (A), namely (B factorB − B factorA)/B factorA. Data in this figure, as well as values for increases in B factors mentioned in the text, are derived from models in which the six Sγ atoms of cysteine residues participating in intrachain disulfide linkages were included in the refinement.
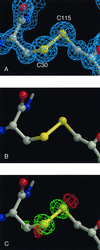
Figure 4
Sequential Fourier maps and difference Fourier maps showing cleavage of the Cys-30–Cys-115 disulfide bond in HEWL as a function of x-ray dose at 1.2-Å resolution. (A) Initial 2Fo-Fc map, contoured at 1.5 σ. (B) Difference Fourier map, wFA − wFB, for two successive data sets collected using an attenuator, contoured at 5 σ (the w refers to
sigmaa
weighting (39). (c) Difference Fourier map, wFB − wFC, for two successive data sets, between which the crystal was exposed to the unattenuated beam, contoured at 6 σ.
Similar articles
- Radiation damage of protein crystals at cryogenic temperatures between 40 K and 150 K.
Teng TY, Moffat K. Teng TY, et al. J Synchrotron Radiat. 2002 Jul 1;9(Pt 4):198-201. doi: 10.1107/s0909049502008579. Epub 2002 Jun 30. J Synchrotron Radiat. 2002. PMID: 12091725 - Evidence for the formation of disulfide radicals in protein crystals upon X-ray irradiation.
Weik M, Bergès J, Raves ML, Gros P, McSweeney S, Silman I, Sussman JL, Houée-Levin C, Ravelli RB. Weik M, et al. J Synchrotron Radiat. 2002 Nov 1;9(Pt 6):342-6. doi: 10.1107/s0909049502014589. Epub 2002 Nov 1. J Synchrotron Radiat. 2002. PMID: 12409620 - Radiation damage and dose limits in serial synchrotron crystallography at cryo- and room temperatures.
de la Mora E, Coquelle N, Bury CS, Rosenthal M, Holton JM, Carmichael I, Garman EF, Burghammer M, Colletier JP, Weik M. de la Mora E, et al. Proc Natl Acad Sci U S A. 2020 Feb 25;117(8):4142-4151. doi: 10.1073/pnas.1821522117. Epub 2020 Feb 11. Proc Natl Acad Sci U S A. 2020. PMID: 32047034 Free PMC article. - Physical and chemical considerations of damage induced in protein crystals by synchrotron radiation: a radiation chemical perspective.
O'Neill P, Stevens DL, Garman EF. O'Neill P, et al. J Synchrotron Radiat. 2002 Nov 1;9(Pt 6):329-32. doi: 10.1107/s0909049502014553. Epub 2002 Nov 1. J Synchrotron Radiat. 2002. PMID: 12409618 Review.
Cited by
- Molecular basis of interchain disulfide-bond formation in BMP-9 and BMP-10.
Schwartze TA, Morosky SA, Rosato TL, Henrickson A, Lin G, Hinck CS, Taylor AB, Olsen SK, Calero G, Demeler B, Roman BL, Hinck AP. Schwartze TA, et al. bioRxiv [Preprint]. 2024 Oct 17:2024.10.14.618187. doi: 10.1101/2024.10.14.618187. bioRxiv. 2024. PMID: 39464140 Free PMC article. Preprint. - Energy Absorption and Beam Damage during Microfocus Synchrotron X-ray Diffraction.
Stanko ŠT, Schawe JEK, Spieckermann F, Eckert J, Löffler JF. Stanko ŠT, et al. J Phys Chem Lett. 2024 Jun 20;15(24):6286-6291. doi: 10.1021/acs.jpclett.4c00497. Epub 2024 Jun 7. J Phys Chem Lett. 2024. PMID: 38848352 Free PMC article. - Crystal structure via fluctuation scattering.
Adams P, Greaves TL, Martin AV. Adams P, et al. IUCrJ. 2024 Jul 1;11(Pt 4):538-555. doi: 10.1107/S2052252524003932. IUCrJ. 2024. PMID: 38842120 Free PMC article. - Identifying and avoiding radiation damage in macromolecular crystallography.
Shelley KL, Garman EF. Shelley KL, et al. Acta Crystallogr D Struct Biol. 2024 May 1;80(Pt 5):314-327. doi: 10.1107/S2059798324003243. Epub 2024 Apr 30. Acta Crystallogr D Struct Biol. 2024. PMID: 38700059 Free PMC article. - A database overview of metal-coordination distances in metalloproteins.
Bazayeva M, Andreini C, Rosato A. Bazayeva M, et al. Acta Crystallogr D Struct Biol. 2024 May 1;80(Pt 5):362-376. doi: 10.1107/S2059798324003152. Epub 2024 Apr 29. Acta Crystallogr D Struct Biol. 2024. PMID: 38682667 Free PMC article.
References
- Blundell T L, Johnson L N. Protein Crystallography. New York: Academic; 1976.
- Sygusch J, Allaire M. Acta Crystallogr A. 1988;44:443–448. - PubMed
- Gonzalez A, Nave C. Acta Crystallogr D. 1994;50:874–877. - PubMed
- Hope H. Acta Crystallogr B. 1988;44:22–26. - PubMed
- Garman E F, Schneider T R. J Appl Crystallogr. 1997;29:211–237.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources