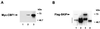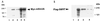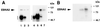A role for SKIP in EBNA2 activation of CBF1-repressed promoters - PubMed (original) (raw)
A role for SKIP in EBNA2 activation of CBF1-repressed promoters
S Zhou et al. J Virol. 2000 Feb.
Abstract
EBNA2 is essential for Epstein-Barr virus (EBV) immortalization of B lymphocytes. EBNA2 functions as a transcriptional activator and targets responsive promoters through interaction with the cellular DNA binding protein CBF1. We have examined the mechanism whereby EBNA2 overcomes CBF1-mediated transcriptional repression. A yeast two-hybrid screen performed using CBF1 as the bait identified a protein, SKIP, which had not previously been recognized as a CBF1-associated protein. Protein-protein interaction assays demonstrated contacts between SKIP and the SMRT, CIR, Sin3A, and HDAC2 proteins of the CBF1 corepressor complex. Interestingly, EBNA2 also interacted with SKIP in glutathione S-transferase affinity and mammalian two-hybrid assays and colocalized with SKIP in immunofluorescence assays. Interaction with SKIP was not affected by mutation of EBNA2 conserved region 6, the CBF1 interaction region, but was abolished by mutation of conserved region 5. Mutation of conserved region 5 also severely impaired EBNA2 activation of a reporter containing CBF1 binding sites. Thus, interaction with both CBF1 and SKIP is necessary for efficient promoter activation by EBNA2. A model is presented in which EBNA2 competes with the SMRT-corepressor complex for contacts on SKIP and CBF1.
Figures
FIG. 1
SKIP interaction with CBF1. (A) GST affinity assay using extract from 293T cells expressing Myc-CBF1. Bound protein was detected by Western blotting using anti-Myc antibody. Extract was incubated with control GST beads (lane 1) or GST-SKIP (lane 2). Lane 3 was loaded with 10 μl of transfected cell extract. (B) Lysate from cells cotransfected with Myc-CBF1 and Flag-SKIP was subjected to immunoprecipitation, and Western blots of the immunoprecipitated proteins were probed with anti-Flag antibody to detect Flag-SKIP. Flag-SKIP coprecipitated with Myc-CBF1 in precipitates formed with rabbit anti-Myc antibody (lane 1). Flag-SKIP was not precipitated by control preimmune rabbit antiserum (lane 2). As a positive control, Flag-SKIP was directly immunoprecipitated by anti-Flag monoclonal antibody (lane 3). Flag-SKIP was not observed in immunoprecipitates generated with an irrelevant monoclonal antibody (anti-Zta; lane 4). Fourfold more extract was used in the coprecipitation than in the direct precipitation. The vertical bar indicates the position of the immunoglobulin heavy chain.
FIG. 2
SKIP interacts with the CBF1 corepressor proteins CIR and HDAC. SKIP-corepressor interactions were analyzed using coimmunoprecipitation, yeast two-hybrid, and mammalian two-hybrid assays. (A) Coimmunoprecipitation assay using extracts of 293T cells cotransfected with CIR-Flag and SKIP. Western blots of the immunoprecipitated proteins were probed with anti-Flag antibody to detect CIR-Flag. Incubation with rabbit anti-SKIP antibody coprecipitated CIR-Flag (lane 1). CIR-Flag was directly precipitated by mouse anti-Flag antibody (lane 2). The amount of extract used in the direct precipitation was one-fourth of that used in the coprecipitation. Lane 3 was loaded with 10 μl of transfected cell extract. (B) SKIP interacts with CIR and HDAC in a yeast two-hybrid assay in which interaction is measured by induction of β-galactosidase activity. Yeast cells were cotransformed with Gal4DBD-SKIP plus Gal4ACT vector (negative control; lane 1), Gal4DBD-CBF1 plus EBNA2(252-425) (positive control; lane 2), Gal4DBD-CIR plus Gal4ACT-SKIP (lane 3), Gal4DBD-SKIP plus Gal4ACT-CIR (lane 4), or Gal4DBD-SKIP plus Gal4ACT-HDAC2 (lane 5). The results shown are an average of three experiments with the standard deviation indicated. (C) Mammalian two-hybrid assay in which Gal4-HDAC2 is targeted to a 5xGal4TK-CAT reporter and the ability of a SKIP activation domain fusion, SKIP-Rta, to activate reporter expression is used as a measure of SKIP-HDAC interaction. HeLa cells were cotransfected with 5xGal4TK-CAT reporter alone or with Gal4-HDAC plus increasing amounts (0, 0.5, 1.0, and 1.5 μg) of SKIP-Rta. For comparison, the 5xGal4TK-CAT reporter was cotransfected with Gal4-CBF1, which represses reporter expression, and Gal4-CBF1 plus EBNA2, which activates the reporter through tethering to CBF1.
FIG. 3
SKIP also interacts with the corepressor proteins Sin3A and SMRT. (A) GST affinity assay in which an extract of Myc-mSin3A-transfected 293T cells was applied to GST-SKIP, GST-CBF1, or control GST beads and a Western blot of the bound proteins was probed with anti-Myc monoclonal antibody. Myc-mSin3A did not bind to GST alone (lane 1) but bound to both GST-CBF1 (lane 2) and GST-SKIP (lane 3). Transfected cell extract (10 μl) (lane 4) and Myc-mSin3A directly immunoprecipitated with anti-Myc monoclonal antibody (lane 5) served as positive controls. (B) Coimmunoprecipitation of Flag-SMRT and SKIP from extracts of cotransfected 293T cells. Flag-SMRT was detected on a Western blot using mouse anti-Flag antibody. Lane 1, precipitation with by preimmune rabbit antibody; lane 2, transfected cell extract (10 μl); lane 3, direct precipitation of Flag-SMRT with anti-Flag antibody; lane 4, coprecipitation of Flag-SMRT with SKIP from extracts incubated with anti-SKIP rabbit antibody. The amount of extract used in the direct precipitation was one-fourth of that used in the coprecipitation.
FIG. 4
Intranuclear colocalization of SKIP and EBNA2 in the presence of HDAC. Immunofluorescence assay in Vero cells cotransfected with EBNA2, HA-SKIP, and HDAC shows that EBNA2 (red) and SKIP (green) each gives a punctate staining pattern that colocalizes in the merged image (yellow). Primary antibodies were anti-EBNA2 mouse antibody and rabbit anti-SKIP antibody. Secondary antibodies were FITC-conjugated donkey anti-rabbit (green) and rhodamine-conjugated goat anti-mouse (red).
FIG. 5
EBNA2 interacts with SKIP in addition to CBF1. (A) Immunoprecipitation assay using extracts from 293T cells transfected with EBNA2 plus Flag-CBF1 to show interaction between EBNA2 and CBF1. EBNA2 was detected by Western blot analysis using anti-EBNA2 mouse monoclonal antibody. Lane 1, rabbit anti-CBF1 antibody-coprecipitated EBNA2; lanes 2 and 6, direct immunoprecipitation of EBNA2 by anti-EBNA2 mouse monoclonal antibody; lanes 3 and 7, transfected cell extract (10 μl); lane 4, precipitation with preimmune rabbit antiserum; lane 5, precipitation with irrelevant mouse monoclonal antibody (anti-CD23). The amount of extract used in the direct precipitation was one-fourth of that used in the coprecipitation. (B) GST affinity assay in which extracts from 293T cells transfected with EBNA2 were incubated with GST (lane 1) or GST-SKIP (lane 2). Transfected cell extract (10 μl) was loaded in lane 3.
FIG. 6
SMRT competes for EBNA2 binding to both CBF1 and SKIP. In mammalian two-hybrid assays, SKIP facilitates EBNA2 interaction with Gal4-CBF1 while SMRT interferes with the ability of EBNA2 to bind to (A) Gal4-CBF1 or (B) Gal4-SKIP and activate expression from a 5xGal4TK-CAT reporter. (A) HeLa cells were cotransfected with 5xGal4TK-CAT reporter, TK-Luciferase control, Gal4-CBF1 alone or in the presence of EBNA2, and increasing amounts of either SKIP or SMRT (0.1, 0.5, and 2 μg) as indicated. SKIP facilitated the EBNA2-CBF1 interaction, while SMRT abolished activation of the reporter by EBNA2. (B) HeLa cells were cotransfected with 5xGal4TK-CAT reporter, TK-Luciferase control, Gal4-SKIP alone or in the presence of EBNA2, and increasing amounts of SMRT (0.1, 0.5, and 2 μg) as indicated. SMRT also abolished reporter activation by SKIP-tethered EBNA2.
FIG. 7
EBNA2 interaction with SKIP is not mediated by the CBF1 interaction domain. Mammalian two-hybrid assay shows that the EBNA2(WW323SR) mutant that does not interact with Gal4-CBF1 retains interaction with Gal4-SKIP. HeLa cells were transfected with a 5xGal4TK-CAT reporter, TK-Luciferase control, Gal4-CBF1 or Gal4-SKIP, and wtEBNA2 (wtE2) or mutant EBNA2 [E2(WW323SR)]. The amount of transfected DNA was equalized using vector DNA. Both Gal4-CBF1 and Gal4-SKIP repress CAT reporter expression. Activation of expression by EBNA2 is indicative of interaction between EBNA2 and Gal4-CBF1 or Gal4-SKIP. E2(WW323SR) activates expression in the presence of Gal4-SKIP but not in the presence of Gal4-CBF1.
FIG. 8
SKIP interaction is necessary for effective EBNA2 targeting of promoter-bound CBF1. Transient expression and mammalian two-hybrid assays linking the inability of EBNA2(II307SR) to efficiently activate 4xCp-CAT with an inability to interact with SKIP. (A) CAT assay performed using extracts of HeLa cells transfected with a 4xCp-CAT reporter alone or in the presence of either wtEBNA2 (wtE2) or the EBNA2 mutant E2(II307SR). (B) Mammalian two-hybrid assay performed in HeLa cells transfected with a 5xGal4TK-CAT reporter, Gal4-SKIP as indicated, and either the CR5 EBNA2 mutant E2(II307SR) or the CR6 mutant E2(PI326SR). Gal4-SKIP represses expression from 5xGal4TK-CAT. This repression is overcome by the E2(PI326SR) mutant but not by the E2(II307SR) mutant, indicating that mutation in CR5 ablates interaction of EBNA2 with SKIP, whereas a CR6 mutant retains SKIP interaction.
FIG. 9
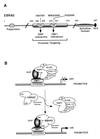
(A) Schematic representation of EBNA2 illustrating the relative locations of characterized functional domains and of the mutants used in this study. The amino acid numbers are indicated. CR5, CR6 (33), and a nuclear localization signal (NLS) (36) are indicated. (B) Model for EBNA2 activation of CBF1-repressed promoters. CBF1 binds to the DNA sequence GTGGGAA in responsive promoters. SKIP is bound to CBF1. SMRT contacts both SKIP and CBF1. SMRT is a component of a corepressor complex that includes Sin3A, SAP30, CIR, HDAC1, and HDAC2 (19, 25), and potentially other Sin3-associated proteins (indicated by x). This complex mediates promoter repression through chromatin remodeling. EBNA2 competes with SMRT for contacts on both SKIP and CBF1. Displacement of the SMRT-corepressor complex relieves repression, and introduction of the EBNA2 activation domain induces transcriptional activation. EBNA2 mutants in CR5 and CR6 lose the ability to interact with SKIP and CBF1, respectively. Loss of either interaction impairs the ability of EBNA2 to activate CBF1-repressed promoters.
Similar articles
- SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC To facilitate NotchIC function.
Zhou S, Fujimuro M, Hsieh JJ, Chen L, Miyamoto A, Weinmaster G, Hayward SD. Zhou S, et al. Mol Cell Biol. 2000 Apr;20(7):2400-10. doi: 10.1128/MCB.20.7.2400-2410.2000. Mol Cell Biol. 2000. PMID: 10713164 Free PMC article. - Nuclear localization of CBF1 is regulated by interactions with the SMRT corepressor complex.
Zhou S, Hayward SD. Zhou S, et al. Mol Cell Biol. 2001 Sep;21(18):6222-32. doi: 10.1128/MCB.21.18.6222-6232.2001. Mol Cell Biol. 2001. PMID: 11509665 Free PMC article. - Epstein-Barr virus BamHi-a rightward transcript-encoded RPMS protein interacts with the CBF1-associated corepressor CIR to negatively regulate the activity of EBNA2 and NotchIC.
Zhang J, Chen H, Weinmaster G, Hayward SD. Zhang J, et al. J Virol. 2001 Mar;75(6):2946-56. doi: 10.1128/JVI.75.6.2946-2956.2001. J Virol. 2001. PMID: 11222720 Free PMC article. - Both Epstein-Barr viral nuclear antigen 2 (EBNA2) and activated Notch1 transactivate genes by interacting with the cellular protein RBP-J kappa.
Strobl LJ, Höfelmayr H, Stein C, Marschall G, Brielmeier M, Laux G, Bornkamm GW, Zimber-Strobl U. Strobl LJ, et al. Immunobiology. 1997 Dec;198(1-3):299-306. doi: 10.1016/s0171-2985(97)80050-2. Immunobiology. 1997. PMID: 9442401 Review. - EBNA2 and Notch signalling in Epstein-Barr virus mediated immortalization of B lymphocytes.
Zimber-Strobl U, Strobl LJ. Zimber-Strobl U, et al. Semin Cancer Biol. 2001 Dec;11(6):423-34. doi: 10.1006/scbi.2001.0409. Semin Cancer Biol. 2001. PMID: 11669604 Review.
Cited by
- SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC To facilitate NotchIC function.
Zhou S, Fujimuro M, Hsieh JJ, Chen L, Miyamoto A, Weinmaster G, Hayward SD. Zhou S, et al. Mol Cell Biol. 2000 Apr;20(7):2400-10. doi: 10.1128/MCB.20.7.2400-2410.2000. Mol Cell Biol. 2000. PMID: 10713164 Free PMC article. - EBV nuclear antigen EBNALP dismisses transcription repressors NCoR and RBPJ from enhancers and EBNA2 increases NCoR-deficient RBPJ DNA binding.
Portal D, Zhao B, Calderwood MA, Sommermann T, Johannsen E, Kieff E. Portal D, et al. Proc Natl Acad Sci U S A. 2011 May 10;108(19):7808-13. doi: 10.1073/pnas.1104991108. Epub 2011 Apr 25. Proc Natl Acad Sci U S A. 2011. PMID: 21518914 Free PMC article. - Mammalian PRP4 kinase copurifies and interacts with components of both the U5 snRNP and the N-CoR deacetylase complexes.
Dellaire G, Makarov EM, Cowger JJ, Longman D, Sutherland HG, Lührmann R, Torchia J, Bickmore WA. Dellaire G, et al. Mol Cell Biol. 2002 Jul;22(14):5141-56. doi: 10.1128/MCB.22.14.5141-5156.2002. Mol Cell Biol. 2002. PMID: 12077342 Free PMC article. - Nuclear localization of CBF1 is regulated by interactions with the SMRT corepressor complex.
Zhou S, Hayward SD. Zhou S, et al. Mol Cell Biol. 2001 Sep;21(18):6222-32. doi: 10.1128/MCB.21.18.6222-6232.2001. Mol Cell Biol. 2001. PMID: 11509665 Free PMC article. - Inhibition of Epstein-Barr virus-induced growth proliferation by a nuclear antigen EBNA2-TAT peptide.
Farrell CJ, Lee JM, Shin EC, Cebrat M, Cole PA, Hayward SD. Farrell CJ, et al. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4625-30. doi: 10.1073/pnas.0306482101. Epub 2004 Mar 19. Proc Natl Acad Sci U S A. 2004. PMID: 15070768 Free PMC article.
References
- Artavanis-Tsakonas S, Rand M D, Lake R J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
- Ayer D E. Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol. 1999;9:193–198. - PubMed
- Dahl R, Wani B, Hayman M J. The Ski oncoprotein interacts with Skip, the human homolog of Drosophila Bx42. Oncogene. 1998;16:1579–1586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases