The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma - PubMed (original) (raw)
The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma
H Haghnegahdar et al. J Leukoc Biol. 2000 Jan.
Abstract
Continuous expression of the MGSA/GROalpha, beta, or gamma chemokine bestows tumor-forming capacity to the immortalized murine melanocyte cell line, melan-a. The mechanism for this transformation is unclear, although both autocrine and paracrine processes are possible because melan-a cells as well as endothelial cells express a low level of the receptor for this ligand. To further define the role of MGSA/GRO proteins in melanocyte transformation, two types of experiments were designed to neutralize the biological effects of MGSA/GRO in the transfected melan-a clones: (1) the effect of neutralizing antiserum to MGSA/GRO proteins on melan-a tumor growth was assessed; (2) the tumor-forming capacity of melan-a clones expressing ELR motif-mutated forms of MGSA/GRO with compromised receptor affinity was compared to the tumor-forming capacity of clones expressing wild-type MGSA/GRO. These experiments revealed that SCID mice inoculated with MGSA/GROalpha- or gamma-expressing melan-a cells and subsequently treated with antiserum to the respective chemokine exhibited decreased tumor growth. This reduction in tumor growth was accompanied by declining angiogenic activity in MGSA/GROgamma-expressing tumors. Moreover, athymic nude mice injected with melan-a cells expressing ELR-mutant forms of MGSA/GROalpha exhibited markedly impaired tumor-forming capacity compared with those mice injected with melan-a clones expressing wild-type MGSA/GRO. These data suggest that continuous expression of MGSA/GRO proteins may facilitate tumor growth by stimulating the growth of microvessels into the tumor (paracrine) and by affecting melanocyte growth (autocrine).
Figures
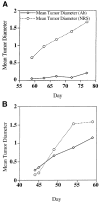
Fig. 1
Effect of antiserum to MGSA/GROα or MGSA/GROγ on the growth of melan-a tumors in SCID mice. Melan-a cells (5 × 106) expressing MGSA/GROγ (A) or MGSA/GROα (B) were injected into 12 young female SCID mice. (A) Mice from each group (expressing MGSA/GROα or γ) were injected every-other day with decomplemented blocking antiserum to these respective chemokines or with heat-inactivated normal rabbit serum. We compared the mean tumor diameter as determined by micrometer measurement in the group injected with antibodies (Ab) to MGSA/GRO or to that of the group injected with normal rabbit serum (NRS). Three mice of the six treated with the antiserum to the MGSA/GROγ slowly developed very small tumors (over 2.5 months; final mean tumor diameter ranged from 0.7 to 0.2 cm for the three out of six mice that developed tumors), whereas those six mice treated with NRS quickly developed very large tumors by 8 weeks after the initial injection of melan-a cells expressing MGSA/GROα (final tumor diameters ranged from 2.3 to 1 cm). (B) For 8.5 weeks antiserum to MGSA/GROα (0.5 mL) or NRS (0.5 mL) was injected every-other day into SCID mice inoculated with MGSA/GROα-expressing melan-a cells (clone mel-a-6). The diameter of the tumors was monitored weekly by micrometer measurement. The MGSA/GROα-expressing cells made tumors in five out of six mice injected with antibody to MGSA/GROα (final tumor diameters ranged from 2.0 to 0.8 cm for the five out of six mice developing tumors) while tumors arose in all six of the mice injected with NRS (final tumor diameters ranged from 2.5 to 1.0 cm).
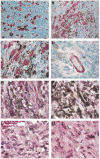
Fig. 2
Immunolocalization of mCXCR2 and histology of the melan-a tumors. Tumors that arose in SCID mice injected with melan-a cells expressing MGSA/GROγ were fixed in paraformaldehyde, embedded in paraffin, sectioned, and stained with antibody to mCXCR2 (Santa Cruz Biotechnology, Inc.) using the ABC Elite kit from Vector Laboratories, with a substrate of aminoethyl carbazole for the peroxidase, which produces a red staining indicative of the presence of the antigen, mCXCR2. (A) Note the presence of a subset of non-melanotic tumor cells that stain strongly for immunoreactive mCXCR2 (original magnification, ×200). (B) The presence of imunoreactive mCXCR2 tumor cells interspersed among highly pigmented tumor cells is shown (original magnification ×200). (C) At higher power (original magnification ×1000) the presence of CXCR2 immunoreactive spindle-shaped tumor cells in the area of a number of highly pigmented tumor cells is shown. (D) The presence of immunoreactive mCXCR2 in the endothelial cells of a blood vessel in the tumor that formed after injection of melan-a cells expressing MGSA/GROγ (original magnification ×1000). Additional capillaries in cross section also exhibit immunoreactivity for the mCXCR2. (E, F) The H & E staining of the MGSA/GROγ-expressing tumors treated with NRS (E) or antiserum to MGSA/GROγ is shown (F) (original magnification ×1000). (G, H) The H & E staining of the MGSA/GROα-expressing tumors treated with NRS (G) or antiserum to MGSA/GROα is shown (H) (original magnification ×1000). Note the typical lack of leukocytic infiltration in these tumors.
Fig. 3
Immunohistochemical staining of tumor-associated blood vessels as visualized by CD31 antisera. (A, B) Heavily pigmented MGSA/GRO tumors: A, received treatment with normal rabbit serum and shows robust in-growth of blood vessels; B, received treatment with MGSA/GRO antisera and shows scant numbers of blood vessels. (C, D) Non-pigmented MGSA/GRO tumors: panel C shows moderate neovascularization after treatment with normal rabbit serum; panel D shows little evidence of neovascularization after treatment with MGSA/GRO antisera.
Similar articles
- MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression.
Wang D, Yang W, Du J, Devalaraja MN, Liang P, Matsumoto K, Tsubakimoto K, Endo T, Richmond A. Wang D, et al. Oncogene. 2000 Sep 21;19(40):4647-59. doi: 10.1038/sj.onc.1203820. Oncogene. 2000. PMID: 11030154 Free PMC article. - Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins.
Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Owen JD, et al. Int J Cancer. 1997 Sep 26;73(1):94-103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. Int J Cancer. 1997. PMID: 9334815 - Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression.
Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Luan J, et al. J Leukoc Biol. 1997 Nov;62(5):588-97. doi: 10.1002/jlb.62.5.588. J Leukoc Biol. 1997. PMID: 9365113 Review. - Effects of MGSA/GRO alpha on melanocyte transformation.
Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Balentien E, et al. Oncogene. 1991 Jul;6(7):1115-24. Oncogene. 1991. PMID: 1861861 - Autocrine and paracrine roles for growth factors in melanoma.
Shih IM, Herlyn M. Shih IM, et al. In Vivo. 1994 Jan-Feb;8(1):113-23. In Vivo. 1994. PMID: 7519892 Review.
Cited by
- IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis.
Wang CQF, Akalu YT, Suarez-Farinas M, Gonzalez J, Mitsui H, Lowes MA, Orlow SJ, Manga P, Krueger JG. Wang CQF, et al. J Invest Dermatol. 2013 Dec;133(12):2741-2752. doi: 10.1038/jid.2013.237. Epub 2013 Apr 30. J Invest Dermatol. 2013. PMID: 23732752 Free PMC article. - GROα regulates human embryonic stem cell self-renewal or adoption of a neuronal fate.
Krtolica A, Larocque N, Genbacev O, Ilic D, Coppe JP, Patil CK, Zdravkovic T, McMaster M, Campisi J, Fisher SJ. Krtolica A, et al. Differentiation. 2011 Apr;81(4):222-32. doi: 10.1016/j.diff.2011.01.001. Epub 2011 Mar 10. Differentiation. 2011. PMID: 21396766 Free PMC article. - Melanoma-derived factors alter the maturation and activation of differentiated tissue-resident dendritic cells.
Hargadon KM, Bishop JD, Brandt JP, Hand ZC, Ararso YT, Forrest OA. Hargadon KM, et al. Immunol Cell Biol. 2016 Jan;94(1):24-38. doi: 10.1038/icb.2015.58. Epub 2015 May 26. Immunol Cell Biol. 2016. PMID: 26010746 - Transcriptome Analysis Provides Insights into the Markers of Resting and LPS-Activated Macrophages in Grass Carp (Ctenopharyngodon idella).
Hu Y, Wei X, Liao Z, Gao Y, Liu X, Su J, Yuan G. Hu Y, et al. Int J Mol Sci. 2018 Nov 12;19(11):3562. doi: 10.3390/ijms19113562. Int J Mol Sci. 2018. PMID: 30424518 Free PMC article.
References
- Koch AE, Polverin PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. - PubMed
- Szekanecz Z, Shah MR, Harlow LA, Pearce WH, Koch AE. Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth. Pathobiol. 1994;62:134–139. - PubMed
- Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van Damme J, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol. 1995;57:752–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA056704-07/CA/NCI NIH HHS/United States
- CA34590/CA/NCI NIH HHS/United States
- R01 CA034590/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- 5P30AR4194/AR/NIAMS NIH HHS/United States
- CA66180/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources