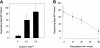A cell-free system for regulated exocytosis in PC12 cells - PubMed (original) (raw)
A cell-free system for regulated exocytosis in PC12 cells
J Avery et al. J Cell Biol. 2000.
Abstract
We have developed a cell-free system for regulated exocytosis in the PC12 neuroendocrine cell line. Secretory vesicles were preloaded with acridine orange in intact cells, and the cells were sonicated to produce flat, carrier-supported plasma membrane patches with attached vesicles. Exocytosis resulted in the release of acridine orange which was visible as a disappearance of labeled vesicles and, under optimal conditions, produced light flashes by fluorescence dequenching. Exocytosis in vitro requires cytosol and Ca(2+) at concentrations in the micromolar range, and is sensitive to Tetanus toxin. Imaging of membrane patches at diffraction- limited resolution revealed that 42% of docked granules were released in a Ca(2+)-dependent manner during 1 min of stimulation. Electron microscopy of membrane patches confirmed the presence of dense-core vesicles. Imaging of membrane patches by atomic force microscopy revealed the presence of numerous particles attached to the membrane patches which decreased in number upon stimulation. Thus, exocytotic membrane fusion of single vesicles can be monitored with high temporal and spatial resolution, while providing access to the site of exocytosis for biochemical and molecular tools.
Figures
Figure 1
Exocytosis of secretory vesicles from membrane patches, monitored by fluorescence dequenching of acridine orange. Cortical fragments prepared from PC12 cells prelabeled with acridine orange were analyzed by fluorescence microscopy. (A) Quantitation of the dequenching signals (by manual counting) in the field of view for fragments perfused with either 10 μM Ca2+ or with control buffer containing 2 mM EGTA. Values are means ± SEM; n = 7, plus Ca2+ and n = 3, control. (B) Ca2+ sensitivity of exocytosis in vitro. Half-maximal stimulation occurred at approximately 2 μM Ca2+. Values are means ± SEM (n = 3).
Figure 2
Cytosol dependence of exocytosis in vitro, measured as in Fig. 1. (A) Membrane patches were prepared in physiological buffer containing the indicated concentrations of rat brain cytosol, before the addition of 10 μM Ca2+. Values are means ± SEM (n = 3). (B) Membrane patches were incubated at 37°C for the times indicated in the presence of cytosol (5 mg/ml), before the addition of Ca2+. Values are means ± SEM (n = 6).
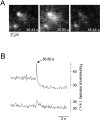
Figure 3
Dequenching flash caused by exocytosis of an acridine-orange–loaded secretory vesicle, imaged by video microscopy at a rate of 25 Hz. (A) Sequence of video images showing a typical dequenching flash arising from a fluorescent spot (left). The delay time after the addition of Ca2+ is indicated. Note that after the flash the spot is largely diminished whereas a second spot (lower right) remains unchanged. (B) Fluorescence intensity profile of the dequenching flash shown in the sequence. A circular mask with a diameter of 10 pixels was centered over both spots. The average intensity was determined for every second frame over the sequence and plotted against time. The blip in the intensity profile of the lower spot is caused by a spillover of acridine orange during exocytosis of the upper spot. a.u., arbitrary units.
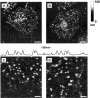
Figure 4
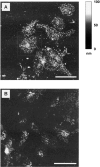
Disappearance of acridine-orange–labeled particles after Ca2+ stimulation. Membrane patches were prepared from cells preloaded with acridine orange and imaged in incubation buffer containing rat brain cytosol and ATP. (A) Low magnification of labeled membrane patches. (B) Same field as in A but labeled at the end of the experiment with FM1-43 in order to visualize all phospholipid membranes. (C–F) High magnification of membrane patches before (C and E) or after a 1-min treatment with 100 μM Ca2+ (D) or control buffer (F).
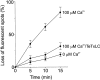
Figure 5
Ca2+-dependent disappearance of labeled particles is blocked by preincubation of the patches with Tetanus toxin light chain. At the times indicated, the patches were imaged and the loss of particles was determined by counting. Note that in this experiment, ATP was added together with Ca2+ and cytosol, and the cytosol concentration was lower than that in the experiment shown in Fig. 4, resulting in a slower time course of exocytosis. Each data point represents the mean value of 15–20 patches obtained from six independent preparations except the 15 min data points for which 4–8 patches were used.
Figure 7
AFM imaging of membrane patches, imaged in air using tapping mode. A and B show two membrane patches representative of those used in the in vitro assay for exocytosis. Boxed areas in A and B are magnified in C and D, respectively. C and D show individual particles. Dotted lines denote the line of cross-section shown above C and D. The mean particle height was 34 ± 0.4 nm and the mean diameter was 225 ± 2 nm (means ± SEM, n = 77). AFM images are black/white-coded with respect to height; a height scale is shown on the right. Bars: (A and B) 2 μm; (C and D) 500 nm.
Figure 6
Cross-sections of membrane patches analyzed by electron microscopy. The fragments were incubated either in the presence of 2 mM EGTA (A) or in the presence of 100 μM Ca2+ (C) for 3 min before fixation. Several secretory vesicles (arrowheads) are attached to the cytoplasmic face of the plasma membrane in the EGTA-treated patch. (B) Two secretory vesicles at higher magnification. Bars: (A and C) 0.5 μm; (B) 0.25 μm.
Figure 8
Incubation in Ca2+-containing buffer causes disappearance of particles as expected for exocytosis. Freshly prepared membrane patches were incubated for 5 min in medium supplemented with either 2 mM EGTA (A) or 10 μM Ca2+ (B) and then fixed as described in Materials and Methods. Fixed samples were imaged in air using tapping-mode AFM. These low-magnification images show two fields each containing ∼10 membrane patches. The boxed area is the same as that shown at higher magnification in Fig. 6 B. The membrane patches in A contain numerous particles on their surfaces. Following incubation in 10 μM Ca2+ (B) the membrane patches have fewer particles, consistent with the disappearance of secretory vesicles due to Ca2+-triggered exocytosis. Bars, 10 μm.
Figure 9
Live AFM imaging of Ca2+-triggered exocytosis. Freshly prepared and unfixed membrane patches were identified by AFM. After the capture of the image shown in A and while the tip was still engaged, Ca2+ (final concentration 10 μM) was gently added at time zero to the imaging buffer. To avoid disrupting the probe, the Ca2+ was added very gently at the edge of the sample and the solution stirred very gently by pipetting up and down. This was to avoid disengaging the probe from the surface. The time delay between Ca2+ addition and exocytosis therefore probably represents the time taken for the diffusing calcium to reach a sufficiently high (threshold) level to trigger exocytosis. The membrane patch in this series of images is visible as a plateau of uniform grey. Taller particles are seen as lighter regions. Between time points B and C a cluster of particles (indicated by the arrows) disappears in response to Ca2+. The dotted lines in B and C denote the line of the cross-sectional height profile shown in F and G, respectively. During the remainder of the time-course neither the shape of the membrane patch nor other surface features are disrupted by repeated scanning. This series of images is representative of the results of 4 experiments. Bar, 2 μm.
Similar articles
- Differential properties of GTP- and Ca(2+)-stimulated exocytosis from large dense core vesicles.
Bai L, Zhu D, Zhou K, Zhou W, Li D, Wang Y, Zhang R, Xu T. Bai L, et al. Traffic. 2006 Apr;7(4):416-28. doi: 10.1111/j.1600-0854.2006.00394.x. Traffic. 2006. PMID: 16536740 - Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells.
Barg S, Olofsson CS, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renström E, Rorsman P. Barg S, et al. Neuron. 2002 Jan 17;33(2):287-99. doi: 10.1016/s0896-6273(02)00563-9. Neuron. 2002. PMID: 11804575 - Regulation of exocytosis in neurons and neuroendocrine cells.
An S, Zenisek D. An S, et al. Curr Opin Neurobiol. 2004 Oct;14(5):522-30. doi: 10.1016/j.conb.2004.08.008. Curr Opin Neurobiol. 2004. PMID: 15464884 Review.
Cited by
- The tetraspanin web revisited by super-resolution microscopy.
Zuidscherwoude M, Göttfert F, Dunlock VM, Figdor CG, van den Bogaart G, van Spriel AB. Zuidscherwoude M, et al. Sci Rep. 2015 Jul 17;5:12201. doi: 10.1038/srep12201. Sci Rep. 2015. PMID: 26183063 Free PMC article. - The extracellular δ-domain is essential for the formation of CD81 tetraspanin webs.
Homsi Y, Schloetel JG, Scheffer KD, Schmidt TH, Destainville N, Florin L, Lang T. Homsi Y, et al. Biophys J. 2014 Jul 1;107(1):100-13. doi: 10.1016/j.bpj.2014.05.028. Biophys J. 2014. PMID: 24988345 Free PMC article. - The Nanoscale Observation of the Three-Dimensional Structures of Neurosynapses, Membranous Conjunctions Between Cultured Hippocampal Neurons and Their Significance in the Development of Epilepsy.
Sun L, Jiang S, Tang X, Zhang Y, Qin L, Jiang X, Yu AC. Sun L, et al. Mol Neurobiol. 2016 Dec;53(10):7137-7157. doi: 10.1007/s12035-015-9588-1. Epub 2015 Dec 17. Mol Neurobiol. 2016. PMID: 26680419 - Identifying Biological and Biophysical Features of Different Maturation States of α-Synuclein Amyloid Fibrils.
Skamris T, Vestergaard B, Madsen KL, Langkilde AE, Foderà V. Skamris T, et al. Methods Mol Biol. 2023;2551:321-344. doi: 10.1007/978-1-0716-2597-2_22. Methods Mol Biol. 2023. PMID: 36310213 - Transparent Electrode Materials for Simultaneous Amperometric Detection of Exocytosis and Fluorescence Microscopy.
Kisler K, Kim BN, Liu X, Berberian K, Fang Q, Mathai CJ, Gangopadhyay S, Gillis KD, Lindau M. Kisler K, et al. J Biomater Nanobiotechnol. 2012;3(2A):243-253. doi: 10.4236/jbnb.2012.322030. J Biomater Nanobiotechnol. 2012. PMID: 22708072 Free PMC article.
References
- Ann K., Kowalchyk J.A., Loyet K.M., Martin T.F.J. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J. Biol. Chem. 1997;272:19637–19640 . - PubMed
- Avery J., Jahn R., Edwardson J.M. Reconstitution of regulated exocytosis in cell-free systemsa critical appraisal. Annu. Rev. Physiol. 1999;61:777–807 . - PubMed
- Baker P.F., Whitaker M.J. Influence of ATP and calcium on the cortical reaction in sea urchin eggs. Nature. 1978;276:513–515 . - PubMed
- Balch W.E., Glick B.S., Rothman J.E. Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 1984;39:525–536 . - PubMed
- Brunk U.T., Dalen H., Roberg K., Hellequist H.B. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 1997;23:616–626 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous