Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics - PubMed (original) (raw)
Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics
M A Dickson et al. Mol Cell Biol. 2000 Feb.
Abstract
Normal human cells exhibit a limited replicative life span in culture, eventually arresting growth by a process termed senescence. Progressive telomere shortening appears to trigger senescence in normal human fibroblasts and retinal pigment epithelial cells, as ectopic expression of the telomerase catalytic subunit, hTERT, immortalizes these cell types directly. Telomerase expression alone is insufficient to enable certain other cell types to evade senescence, however. Such cells, including keratinocytes and mammary epithelial cells, appear to require loss of the pRB/p16(INK4a) cell cycle control mechanism in addition to hTERT expression to achieve immortality. To investigate the relationships among telomerase activity, cell cycle control, senescence, and differentiation, we expressed hTERT in two epithelial cell types, keratinocytes and mesothelial cells, and determined the effect on proliferation potential and on the function of cell-type-specific growth control and differentiation systems. Ectopic hTERT expression immortalized normal mesothelial cells and a premalignant, p16(INK4a)-negative keratinocyte line. In contrast, when four keratinocyte strains cultured from normal tissue were transduced to express hTERT, they were incompletely rescued from senescence. After reaching the population doubling limit of their parent cell strains, hTERT(+) keratinocytes entered a slow growth phase of indefinite length, from which rare, rapidly dividing immortal cells emerged. These immortal cell lines frequently had sustained deletions of the CDK2NA/INK4A locus or otherwise were deficient in p16(INK4a) expression. They nevertheless typically retained other keratinocyte growth controls and differentiated normally in culture and in xenografts. Thus, keratinocyte replicative potential is limited by a p16(INK4a)-dependent mechanism, the activation of which can occur independent of telomere length. Abrogation of this mechanism together with telomerase expression immortalizes keratinocytes without affecting other major growth control or differentiation systems.
Figures
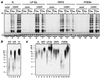
FIG. 1
Telomerase activity and telomere maintenance in hTERT transductants. (a) TRAP assays of telomerase activity in control (vector) and hTERT-transduced cell lines tested at indicated PD levels. The group of three lanes designated by each number show assay results for 2 μg of extract preincubated with RNase as a control and for 1 and 2 μg of extract assayed. Groups: 1, N/BABE-2F, 50 PD; 2, N/TERT-2F, 48 PD (pre-SGP); 3, N/TERT-1, 57 PD (early SGP); 4, LiF-Ep/BABE-1, 35 PD; 5, LiF-Ep/BABE-2, 31 PD; 6, LiF-Ep/TERT-2, 39 PD (late SGP); 7, LiF-Ep/TERT-1, 45 PD (RDI); 8, OKF6, 18 PD; 9, OKF6/BABE-1, 39 PD; 10, OKF6/TERT-1, 42 PD (beginning of SGP); 11, OKF6/TERT-1, 60 PD (early RDI); 12, OKF6/TERT-1, 107 PD; 13, POE9n/BABE-1, 32 PD; 14, POE9n/BABE-1, 83 PD; 15, POE9n/TERT-1, 38 PD; 16, POE9n/TERT-1, 127 PD. HT, reaction mixture heat treated before thermocycling; IC, internal (positive) control amplification product showing activity of PCR reagents.
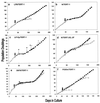
FIG. 2
Long-term replication characteristics of control and hTERT-expressing cell lines. Early- to mid-life-span cultures of LP9 (a), N (b and d), LiF-Ep (c), OKF6 (e), and POE9n (f) were transduced with control or hTERT expression vectors (time of transduction indicated by large arrow), were drug selected, and then were serially passaged. Graphs show passage history of the parent cell line before transduction (○), of control vector transductants (▵), and of hTERT transductants (■). The label in each panel is the name of the immortal cell line that resulted from that hTERT transduction. In panel c, +, +,−, and − indicate the appearance of p53-negative cells in the LiF-Ep/TERT-1 population during serial passage, as described in the text (+, 100 of 100 colonies examined were p53-positive; +,−, 51 of 100 colonies examined were p53 positive and 49 of 100 were p53 negative; −, 99 of 100 colonies examined were p53 negative). In panel d, after transduction with control or hTERT vector, half of each drug-selected population was serially passaged in K-sfm (▴, N/BABE-2G; ■, N/TERT-2G) and half was serially passaged in the feeder cell-FAD system (▵, N/BABE-2F; □, N/TERT-2F). In panel e, some OKF6/TERT-1 cells cryopreserved at the onset of SGP (indicated by *) were thawed and the serial passage was repeated (□), yielding another RDI line designated OKF6/TERT-1R. Panel f includes the replication history of POE-19, a line of apparently normal oral keratinocytes cultured from the same donor as POE9n. The short life span (∼20 PD) of POE-19 indicates that the long (∼85-PD) life span of POE9n and POE9n/BABE is abnormally extended, as discussed in the text.
FIG. 3
Expression of cell cycle regulatory proteins by hTERT transductants. (a) Western blots comparing parent cell lines with hTERT-immortalized cell lines. “RDI+_n_” indicates number of population doublings after RDI cells emerged from the SGP population. Lanes: 1, N, 37 PD (mid-life span); 2, N/TERT-1, 135 PD (RDI+68); 3, LiF-Ep, 20 PD (mid-life span); 4, LiF-Ep/TERT-1, 90 PD (RDI+55); 5, OKF6, 19 PD (mid-life span); 6, OKF6/TERT-1, 63 PD (RDI+11); 7, OKF6/TERT-2, 52 PD (RDI+15). The mobility of the protein recognized by each antibody was as expected for the protein, compared with molecular mass markers separated on the same gel (data not shown). (b) Western blot analysis of p16INK4a expression by hTERT-immortalized mesothelial cells and fibroblasts. Life span designations as noted above. Lanes 1, strain N keratinocytes, 33 PD (mid-life span); 2, LP9 mesothelial cells, 41 PD (mid-life span); 3, LP9/TERT-1, 108 PD; 4, S1F/BABE-1, 70 PD (late life span); 5, S1F/TERT-1, 147 PD.
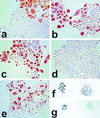
FIG. 4
Immunocytochemical staining of cell lines for p16INK4a (a to e, phase contrast) and p53 (f and g, bright field). Cells were plated at clonal density in K-sfm and cultured for 4 to 7 days before staining. Life span designations are as for Fig. 3a. (a) N, 28 PD (mid-life span); (b) N/BABE-2G, 55 PD (near senescence); (c) N/TERT-2F, 80 PD (SGP); (d) N/TERT-1, 114 PD (RDI+43); (e) LiF-Ep/TERT-1, 67 PD (RDI+28); (f) LiF-Ep/TERT-1, 39 PD (early RDI); (g) LiF-Ep/TERT-1, 48 PD. Note the heterogeneity of p16 expression in normal N cells in panels a and b, confined to large nondividing cells, with small proliferating cells expressing no detectable p16 whether at mid-life span or in rare dividing cells in the nearly senescent population. As shown in panel c, hTERT-transduced keratinocytes continue the normal pattern of p16 expression during SGP. Some RDI lines that emerge from SGP retain the normal pattern of p16 expression, while some cease expressing p16. The p53+ colonies in panel f are representative of >99% of colonies in the early RDI LiF-Ep/TERT-1 population (as confirmed by the Western blot shown in Fig. 3b). In panel g, the p53+ colony at the left and the p53− colony at the right are representative of the mixed nature of the LiF-Ep/TERT-1 RDI population three passages (9 PD) later.
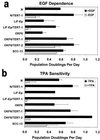
FIG. 5
Long-term retention of normal keratinocyte growth control mechanisms by many, but not all, hTERT-transductants. Cells were plated at low density in K-sfm medium, either in the presence (solid bar) or absence (stippled bar) of EGF (a) or in the presence (stippled bar) or absence (solid bar) of TPA (b), and counted 7 to 9 days later; growth under these conditions calculated as PD per day. Results shown are average of two experiments (standard error shown) except for LiF-Ep, LiF-Ep/TERT-1, and OKF6/TERT-2 in panel b, for which other experiments under slightly different conditions confirmed their TPA sensitivity (data not shown). PD levels of cells assayed: N, 50 PD (late life span); N/TERT-1, 135 P (RDI+68); LiF-Ep, 24 PD (mid-life span); LiF-Ep/TERT-1, 90 PD (RDI+55); OKF6, 37 PD (late life span); OKF6/TERT-1, 97 PD (RDI+45); OKF6/TERT-2, 115 PD (RDI+56); SCC-13 (∼50 PD).
FIG. 6
Differentiation-related protein expression and epithelial tissue formation by hTERT-immortalized keratinocytes. Cells were cultured on conventional plastic dishes for 7 to 9 days in K-sfm (a and b) or in the feeder cell-FAD system (c and d) or were seeded on collagen gels to generate organotypic cultures (e to j), one of which was then grafted to the skin of an athymic mouse (h). Panels a (LiF-Ep, 28 PD) and b (LiF-Ep/TERT-1, 66 PD [RDI+43]) were immunostained for the suprabasal epidermal differentiation keratin K10. Panels c (OKF6, 30 PD) and d (OKF6/TERT-2, 122 PD [RDI+63]) were immunostained for the keratinocyte terminal differentiation protein involucrin. (e) N, 37 PD (mid-life span); f, N/TERT-1, 91 PD (RDI+24); g, LiF-Ep/TERT-1, 90 PD (RDI+55); h, a replicate organotypic culture of the one shown in panel g, 42 days after transplantation as a skin graft to an athymic mouse; i and j, OKF6/TERT-2, 86 PD (RDI+47). Panels e to i were stained with H&E (note that the intense blue of the cell layers below the stratum corneum in the epithelium shown in panel h is the result of a slightly different staining formulation and has no biological significance). Panel j was immunostained for keratin K13. Arrowheads point to the basal layer of the organotypic epithelium, showing that K13 expression begins several cell layers above the first suprabasal layer.
Similar articles
- A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status.
Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricalà C, O'Toole KM. Rheinwald JG, et al. Mol Cell Biol. 2002 Jul;22(14):5157-72. doi: 10.1128/MCB.22.14.5157-5172.2002. Mol Cell Biol. 2002. PMID: 12077343 Free PMC article. - Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation.
Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B, von Werder A, Enders GH, Opitz OG, Rustgi AK. Harada H, et al. Mol Cancer Res. 2003 Aug;1(10):729-38. Mol Cancer Res. 2003. PMID: 12939398 - Genetic and epigenetic changes in human epithelial cells immortalized by telomerase.
Farwell DG, Shera KA, Koop JI, Bonnet GA, Matthews CP, Reuther GW, Coltrera MD, McDougall JK, Klingelhutz AJ. Farwell DG, et al. Am J Pathol. 2000 May;156(5):1537-47. doi: 10.1016/S0002-9440(10)65025-0. Am J Pathol. 2000. PMID: 10793065 Free PMC article. - Molecular changes accompanying senescence and immortalization of cultured human mammary epithelial cells.
Yaswen P, Stampfer MR. Yaswen P, et al. Int J Biochem Cell Biol. 2002 Nov;34(11):1382-94. doi: 10.1016/s1357-2725(02)00047-x. Int J Biochem Cell Biol. 2002. PMID: 12200033 Review. - The human melanocyte: a model system to study the complexity of cellular aging and transformation in non-fibroblastic cells.
Bandyopadhyay D, Timchenko N, Suwa T, Hornsby PJ, Campisi J, Medrano EE. Bandyopadhyay D, et al. Exp Gerontol. 2001 Aug;36(8):1265-75. doi: 10.1016/s0531-5565(01)00098-5. Exp Gerontol. 2001. PMID: 11602203 Review.
Cited by
- Essential role of the histone lysine demethylase KDM4A in the biology of malignant pleural mesothelioma (MPM).
Lapidot M, Case AE, Weisberg EL, Meng C, Walker SR, Garg S, Ni W, Podar K, Hung YP, Carrasco RD, Knott A, Gokhale PC, Sharma S, Pozhitkov A, Kulkarni P, Frank DA, Salgia R, Griffin JD, Saladi SV, Bueno R, Sattler M. Lapidot M, et al. Br J Cancer. 2021 Aug;125(4):582-592. doi: 10.1038/s41416-021-01441-7. Epub 2021 Jun 4. Br J Cancer. 2021. PMID: 34088988 Free PMC article. - hTERT-Driven Immortalization of RDEB Fibroblast and Keratinocyte Cell Lines Followed by Cre-Mediated Transgene Elimination.
Evtushenko NA, Beilin AK, Dashinimaev EB, Ziganshin RH, Kosykh AV, Perfilov MM, Rippa AL, Alpeeva EV, Vasiliev AV, Vorotelyak EA, Gurskaya NG. Evtushenko NA, et al. Int J Mol Sci. 2021 Apr 7;22(8):3809. doi: 10.3390/ijms22083809. Int J Mol Sci. 2021. PMID: 33916959 Free PMC article. - IL-4 impairs wound healing potential in the skin by repressing fibronectin expression.
Serezani APM, Bozdogan G, Sehra S, Walsh D, Krishnamurthy P, Sierra Potchanant EA, Nalepa G, Goenka S, Turner MJ, Spandau DF, Kaplan MH. Serezani APM, et al. J Allergy Clin Immunol. 2017 Jan;139(1):142-151.e5. doi: 10.1016/j.jaci.2016.07.012. Epub 2016 Aug 20. J Allergy Clin Immunol. 2017. PMID: 27554818 Free PMC article. - Establishment and characterization of a telomerase immortalized human gingival epithelial cell line.
Moffatt-Jauregui CE, Robinson B, de Moya AV, Brockman RD, Roman AV, Cash MN, Culp DJ, Lamont RJ. Moffatt-Jauregui CE, et al. J Periodontal Res. 2013 Dec;48(6):713-21. doi: 10.1111/jre.12059. Epub 2013 Feb 27. J Periodontal Res. 2013. PMID: 23441958 Free PMC article. - New signaling pathways govern the host response to C. albicans infection in various niches.
Liu Y, Shetty AC, Schwartz JA, Bradford LL, Xu W, Phan QT, Kumari P, Mahurkar A, Mitchell AP, Ravel J, Fraser CM, Filler SG, Bruno VM. Liu Y, et al. Genome Res. 2015 May;25(5):679-89. doi: 10.1101/gr.187427.114. Epub 2015 Apr 9. Genome Res. 2015. PMID: 25858952 Free PMC article.
References
- Bahuau M, Vidaud D, Jenkins R B, Bieche I, Kimmel D W, Assouline B, Smith J S, Alderete B, Cayuela J M, Harpey J P, Caille B, Vidaud M. Germ-line deletion involving the INK4 locus in familial proneness to melanoma and nervous system tumors. Cancer Res. 1998;58:2298–2303. - PubMed
- Bodnar A G, Ouelette M, Frolkis M, Holt A E, Chiu C-P, Morin G B, Harley C B, Shay J W, Lichsteiner S, Wright W E. Extension of lifespan by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DE012467/DE/NIDCR NIH HHS/United States
- P30 AR042689/AR/NIAMS NIH HHS/United States
- P01 DE12467/DE/NIDCR NIH HHS/United States
- P30 AR42689/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous