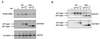A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling - PubMed (original) (raw)
A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling
G A Rodrigues et al. Mol Cell Biol. 2000 Feb.
Abstract
The Gab1 protein is tyrosine phosphorylated in response to various growth factors and serves as a docking protein that recruits a number of downstream signaling proteins, including phosphatidylinositol 3-kinase (PI-3 kinase). To determine the role of Gab1 in signaling via the epidermal growth factor (EGF) receptor (EGFR) we tested the ability of Gab1 to associate with and modulate signaling by this receptor. We show that Gab1 associates with the EGFR in vivo and in vitro via pTyr sites 1068 and 1086 in the carboxy-terminal tail of the receptor and that overexpression of Gab1 potentiates EGF-induced activation of the mitogen-activated protein kinase and Jun kinase signaling pathways. A mutant of Gab1 unable to bind the p85 subunit of PI-3 kinase is defective in potentiating EGFR signaling, confirming a role for PI-3 kinase as a downstream effector of Gab1. Inhibition of PI-3 kinase by a dominant-interfering mutant of p85 or by Wortmannin treatment similarly impairs Gab1-induced enhancement of signaling via the EGFR. The PH domain of Gab1 was shown to bind specifically to phosphatidylinositol 3,4,5-triphosphate [PtdIns(3,4,5)P3], a product of PI-3 kinase, and is required for activation of Gab1-mediated enhancement of EGFR signaling. Moreover, the PH domain mediates Gab1 translocation to the plasma membrane in response to EGF and is required for efficient tyrosine phosphorylation of Gab1 upon EGF stimulation. In addition, overexpression of Gab1 PH domain blocks Gab1 potentiation of EGFR signaling. Finally, expression of the gene for the lipid phosphatase PTEN, which dephosphorylates PtdIns(3,4, 5)P3, inhibits EGF signaling and translocation of Gab1 to the plasma membrane. These results reveal a novel positive feedback loop, modulated by PTEN, in which PI-3 kinase functions as both an upstream regulator and a downstream effector of Gab1 in signaling via the EGFR.
Figures
FIG. 1
Association of Gab1 with the EGFR. (A and B) Anti-Gab1 antibodies (A) and a GST-Gab1 MBD fusion protein (B), respectively, were used to precipitate proteins from lysates prepared from untreated (−) or EGF-stimulated (+) (100 ng/ml for 5 min) NIH 3T3 cells expressing the EGFR (HER14 cells). Coprecipitating proteins were resolved by SDS-PAGE and immunoblotted with either anti-pTyr, anti-EGFR, or anti-Gab1 antibodies. (C) Lysates prepared from untreated or EGF-stimulated (100 ng/ml for 5 min) HER14 cells and NIH 3T3 cells expressing the CD63 mutant were incubated with anti-Gab1 antibodies. Immunocomplexes were resolved by SDS-PAGE and immunoblotted with anti-pTyr antibodies. (D) Lysates prepared from A431 cells were incubated with a GST-Gab1 MBD fusion protein in the presence of 10 mM concentrations of peptides corresponding to EGFR sequences containing a particular tyrosine phosphorylation site. Complexes were resolved by SDS-PAGE and immunoblotted with anti-pTyr antibodies (top) or anti-EGFR antibodies (bottom).
FIG. 2
Potentiation of EGFR-mediated activation of MAPK and JNK by overexpression of Gab1. 293T cells were transiently transfected with increasing amounts (0.5, 2, and 4 μg) of Gab1 expression vector and a constant amount (100 ng) of EGFR (four rightmost lanes) and HA-tagged Erk2 (left) or HA-tagged JNK1 (right) (1.5 μg each). At 48 h posttransfection, cells were lysed and kinase assays were performed as described in Materials and Methods (top blots). Expression of the transfected proteins was determined by immunoblotting total cell lysates with the antibodies indicated beside the lower rows of blots.
FIG. 3
Enhancement of EGFR-mediated activation of JNK by Gab1 requires PI-3 kinase. As shown on the left, an expression vector encoding an HA-tagged dominant-negative mutant of PI-3 kinase (p85INT) (2 μg in the third lane from the left and 4 μg in the fourth lane from the left) was cotransfected with the EGFR, Gab1, and Flag-tagged JNK 1. Anti-Flag immunoprecipitates were divided and one half was used in a JNK assay (top) and the other half was blotted with anti-Flag antibodies to show equal amounts of JNK 1 (bottom). For the two middle blots, total cell lysates were blotted with anti-Gab1 and anti-HA antibodies. As shown on the right, wild-type Gab1 (1.5 μg 3 μg in the second end third lanes from the left, respectively) and a mutant of Gab1 (3 μg) (last lane) containing substitutions of the three putative p85 binding sites (Y448, Y473, and Y590) were introduced with the EGFR and HA-JNK 1, and their relative ability to enhance EGFR activation of this kinase was measured in vitro as described in Materials and Methods.
FIG. 4
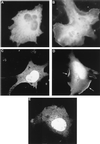
Gab1 is translocated to the plasma membrane in response to EGF stimulation. (A and B) COS1 cells fixed and analyzed by fluorescence microscopy after a cDNA encoding the GFP-Gab1 fusion protein was transfected into them. At 48 h posttransfection, the cells were left untreated (A) or stimulated with 100 ng of EGF per ml (B). (C to E) COS-1 cells fixed and analyzed by confocal fluorescence microscopy after a cDNA encoding the GFP-Gab1 PH domain fusion protein was transfected into them. At 48 h posttransfection, the cells were left untreated (C), stimulated with 100 ng of EGF per ml (D), or pretreated with 100 nM Wortmannin for 10 min prior to stimulation with EGF (E). Arrows indicate fluorescence staining of the plasma membrane. Magnification, ×660.
FIG. 5
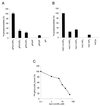
Lipid binding specificity of the Gab1 PH domain. (A) GST-agarose beads containing the Gab1 PH domain were incubated with a deacylated lipid extract prepared from [3H]inositol-labeled IMR33 fibroblasts. The beads were washed, and the bound glycerophosphoinositols were eluted with 0.1 N HCl separated by HPLC and quantitated by liquid scintillation counting. The amount of radioactivity of each compound was calculated as a percentage of the total radioactivity present in the extract and then represented as a percentage relative to the amount of gPI(3,4,5)P3 bound. The error bars represent the standard deviations of three independent experiments. (B) A pool of equal amounts of soluble lipids were mixed with GST-agarose beads containing the Gab1 PH domain, and the bound lipids were analyzed by HPLC. The amount of radioactivity of each compound was corrected for minor differences of the starting material present in the pool and then represented as a percentage relative to the amount of Ins(1,3,4,5)P4 bound. Results are the means of three independent experiments. (C) Displacement of binding of the Gab1 PH binding to PtdIns(3,4,5)P3 by the soluble head group Ins(1,3,4,5)P4. [32P]gPI(3,4,5)P3 was generated in vitro by using PI-3 kinase and PtdIns(4,5)P2 as a substrate and bound to the Gab1 PH domain in the presence of increasing concentrations of Ins(1,3,4,5)P4. gPI(3,4,5)P3, glycerophosphoinositol(3,4,5)phosphate.
FIG. 6
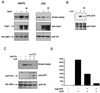
The PH domain of Gab1 is required for signaling. (A) HeLa cells were transfected with increasing amounts (0.5 and 2 μg) of Gab1 or Gab1ΔPH expression vector and HA-tagged JNK 1 (1.5 μg). At 48 h posttransfection cells were stimulated with EGF (100 ng/ml) for 5 min and lysed, and kinase assays were performed as described in Materials and Methods (top blot). Expression levels of the transfected components was determined by immunoblotting of total cell lysates with the indicated antibodies (lower blots). (B) HeLa cells were transfected with increasing amounts (0.5, 1.5, and 3 μg) of Gab1 or Gab1ΔPH expression vector. At 48 h posttransfection cells were stimulated with EGF (100 ng/ml) for 5 min and lysed, and immunoblots of anti-Gab1 immunoprecipitates were conducted using antiphosphotyrosine (top blot) or anti-Gab1 (bottom blot) antibodies.
FIG. 7
Wortmannin and the Gab1 PH domain inhibit Gab1 potentiation of EGFR signaling. (A) An expression vector for EGFR was transfected into 293T cells together with vectors encoding HA-Erk2 (right) or HA-JNK 1 (left) with (second and third lanes from left) or without (leftmost lane) Gab1. At 48 h posttransfection untreated cells or cells pretreated with 100 nM Wortmannin were lysed and kinase assays were performed following immunoprecipitation with anti-HA antibodies (top blot). Immunoblots were performed with the indicated antibodies on total cell lysates (lower blots). (B) HeLa cells were transfected with vectors encoding Gab1. At 48 h posttransfection untreated cells or cells pretreated with 100 nM Wortmannin were lysed and immunoblots were performed using antiphosphotyrosine antibodies (top blot) or anti-Gab1 antibodies (bottom blot) following immunoprecipitation with anti-Gab1 antibodies. (C) HeLa cells were transfected with vectors encoding HA-JNK 1 and increasing concentrations of the Gab1 PH domain. Cells not stimulated (leftmost lane) or stimulated with EGF (100 ng/ml) for 5 min (three rightmost lanes) and then lysed 48 h posttransfection. A JNK assay was performed as described in Materials and Methods following immunoprecipitation with anti-HA antibodies (top blot). Immunoblots of total cell lysates with the indicated antibodies are shown in the lower blots. (D) Results from panel C were quantitated by densitometric scanning of the kinase assay and plotted. The vertical scale is the measurement of the density of the bands in arbitrary units.
FIG. 8
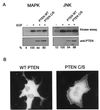
PTEN regulates Gab translocation and phosphorylation. (A) HeLa cells were transfected with a Gab1 expression vector and HA-tagged ERK2 (left) or HA-tagged JNK 1 (right) with either the wild-type or a phosphatase-negative PTEN (PTEN C/S) expression vector. At 48 h posttransfection, cells were untreated (rightmost lanes) or stimulated with EGF (100 ng/ml) for 5 min (three rightmost lanes) and lysed, and kinase assays were performed as described in Materials and Methods. Expression levels of PTEN were determined by immunoblotting of total cell lysates (lower blots). The kinase assay was quantitated by densitometric scanning, and the percentages, relative to the amount of EGF-stimulated activity, are indicated below each lane. (B) GFP-Gab1 expression vector and a fivefold excess of either wild-type or mutant PTEN were transfected into COS-1 cells. At 48 h posttransfection the cells were stimulated with 100 ng of EGF per ml, fixed, and analyzed by fluorescence microscopy.
FIG. 9
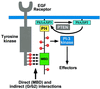
Model outlining the role of Gab1 in receptor tyrosine kinase signaling. Gab1 is recruited to the plasma membrane in response to growth factor stimulation through the concerted action of binding of its MBD to the EGFR directly and through Grb2 and binding of its PH domain to the products of PI-3 kinase. Tyrosine phosphorylation of Gab1 by EGFR leads to recruitment and activation of signaling molecules, including PI-3 kinase. Activation of PI-3 kinase in turn leads to the generation of phosphoinositides that enhance downstream signaling as well as initiate a positive feedback by recruiting more Gab1 molecules to the EGFR. Conversion of PtdIns(3,4,5)P3 to PtdIns(4,5)P2 by PTEN allows for dissociation of Gab1 from the plasma membrane and termination of signaling through Gab1.
Similar articles
- ERK negatively regulates the epidermal growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase.
Yu CF, Liu ZX, Cantley LG. Yu CF, et al. J Biol Chem. 2002 May 31;277(22):19382-8. doi: 10.1074/jbc.M200732200. Epub 2002 Mar 14. J Biol Chem. 2002. PMID: 11896055 - Role of the Grb2-associated binder 1/SHP-2 interaction in cell growth and transformation.
Holgado-Madruga M, Wong AJ. Holgado-Madruga M, et al. Cancer Res. 2004 Mar 15;64(6):2007-15. doi: 10.1158/0008-5472.can-03-2886. Cancer Res. 2004. PMID: 15026337 - Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells.
Kapoor GS, Zhan Y, Johnson GR, O'Rourke DM. Kapoor GS, et al. Mol Cell Biol. 2004 Jan;24(2):823-36. doi: 10.1128/MCB.24.2.823-836.2004. Mol Cell Biol. 2004. PMID: 14701753 Free PMC article. - Regulation of the PTEN phosphatase.
Gericke A, Munson M, Ross AH. Gericke A, et al. Gene. 2006 Jun 7;374:1-9. doi: 10.1016/j.gene.2006.02.024. Epub 2006 Mar 14. Gene. 2006. PMID: 16675164 Review. - Phosphatidylinositol 3-kinase and the control of endosome dynamics: new players defined by structural motifs.
Corvera S. Corvera S. Traffic. 2001 Dec;2(12):859-66. doi: 10.1034/j.1600-0854.2001.21201.x. Traffic. 2001. PMID: 11737823 Review.
Cited by
- Exploration of the shared gene signatures and molecular mechanisms between Alzheimer's disease and intracranial aneurysm.
Liu JY, Yin X, Dong YT. Liu JY, et al. Sci Rep. 2024 Oct 19;14(1):24628. doi: 10.1038/s41598-024-75694-6. Sci Rep. 2024. PMID: 39427050 Free PMC article. - The LCLAT1/LYCAT acyltransferase is required for EGF-mediated phosphatidylinositol-3,4,5-trisphosphate generation and Akt signaling.
Chan V, Camardi C, Zhang K, Orofiamma LA, Anderson KE, Hoque J, Bone LN, Awadeh Y, Lee DKC, Fu NJ, Chow JTS, Salmena L, Stephens LR, Hawkins PT, Antonescu CN, Botelho RJ. Chan V, et al. Mol Biol Cell. 2024 Sep 1;35(9):ar118. doi: 10.1091/mbc.E23-09-0361. Epub 2024 Jul 18. Mol Biol Cell. 2024. PMID: 39024272 Free PMC article. - Dysregulated Gab1 signalling in triple negative breast cancer.
Bongartz H, Mehwald N, Seiß EA, Schumertl T, Naß N, Dittrich A. Bongartz H, et al. Cell Commun Signal. 2024 Mar 6;22(1):161. doi: 10.1186/s12964-024-01542-9. Cell Commun Signal. 2024. PMID: 38448989 Free PMC article. - The Role of GAB1 in Cancer.
Pérez-Baena MJ, Cordero-Pérez FJ, Pérez-Losada J, Holgado-Madruga M. Pérez-Baena MJ, et al. Cancers (Basel). 2023 Aug 20;15(16):4179. doi: 10.3390/cancers15164179. Cancers (Basel). 2023. PMID: 37627207 Free PMC article. Review. - TMEM25 inhibits monomeric EGFR-mediated STAT3 activation in basal state to suppress triple-negative breast cancer progression.
Bi J, Wu Z, Zhang X, Zeng T, Dai W, Qiu N, Xu M, Qiao Y, Ke L, Zhao J, Cao X, Lin Q, Chen XL, Xie L, Ouyang Z, Guo J, Zheng L, Ma C, Guo S, Chen K, Mo W, Fu G, Zhao TJ, Wang HR. Bi J, et al. Nat Commun. 2023 Apr 24;14(1):2342. doi: 10.1038/s41467-023-38115-2. Nat Commun. 2023. PMID: 37095176 Free PMC article.
References
- Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. - PubMed
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B. Curr Biol. 1997;7:261–269. - PubMed
- Bardelli A, Longati P, Gramaglia D, Stella M C, Comoglio P M. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15:3103–3111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous