A developmental change in NMDA receptor-associated proteins at hippocampal synapses - PubMed (original) (raw)
A developmental change in NMDA receptor-associated proteins at hippocampal synapses
N Sans et al. J Neurosci. 2000.
Abstract
The membrane-associated guanylate kinases [Chapsyn-110/postsynaptic density-93 (PSD-93), synapse-associated protein-90 (SAP-90)/PSD-95, and SAP-102] are believed to cluster and anchor NMDA receptors at the synapse and to play a role in signal transduction. We have investigated the developmental changes in expression of these proteins in rat hippocampus using biochemical analyses and quantitative immunogold electron microscopy. At postnatal day 2 (P2), SAP-102 was highly expressed, whereas PSD-93 and PSD-95 were low. SAP-102 expression increased during the first week, stayed stable through P35, and showed a reduced expression at 6 months. From P2 through 6 months, PSD-93 and PSD-95 increased. For PSD-95, the percent of labeled synapses increased almost threefold with age, whereas the number of gold particles per labeled synapse did not change significantly, suggesting that the increase in PSD-95 is attributable primarily to an increase in the number of synapses containing PSD-95. In contrast, for SAP-102, both percent labeled synapses and the number of gold particles per labeled synapse decreased during this time. From Western blots of hippocampus and immunogold analysis of CA1 synapses, the high expression of NR2B at P2 coincides with the high level of SAP-102 at synapses, whereas the later expression of NR2A coincides with that of PSD-93 and PSD-95. To determine whether the changes in PSD-93/95 and SAP-102 reflect preferred associations with NR2A and NR2B, respectively, we measured co-immunoprecipitation in the adult hippocampus. These studies suggest that there is a preference for complexes of NR2A/PSD-93/95 and NR2B/SAP-102. These results indicate that individual receptor-associated proteins may have specific functions that are critical to synapse development.
Figures
Fig. 1.
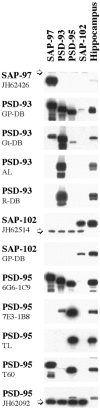
Specificity of antisera to MAGUKs. Western blot analysis reveals that the SAP-97 antiserum does not cross-react with the other PDZ proteins and gives a band corresponding to ∼120 kDa in HEK293 transfected cells. As shown previously (Müller et al., 1995), SAP-97 is not heavily expressed in hippocampus. The anti-rabbit PSD-93 polyclonal antibodies (AL and R-DB) show the same specificity for PSD-93 in transfected cells and in adult hippocampus. In rat hippocampus, at least two bands at ∼103–110 kDa can be detected. By similar analysis, the SAP-102 (JH62514 or GP-DB) antibodies recognize their antigens but show no cross-reactivity with the other members of the MAGUK family. PSD-95 (TL and JH62092) antibodies are the most specific antibodies against PSD-95 available. T60 anti-PSD-95 is a polyclonal antibody that reacts with PSD-95 as strong as with SAP-97 in transfected cells. In hippocampus, in addition to the 95 kDa specific band, we find two additional lighter bands at ∼170 and 85 kDa. The_arrows_ indicate a nonspecific band which appears only in transfected cells. AL, Alomone Laboratories;GP, guinea pig; Gt, goat;R, rabbit; TL, Transduction Laboratories.
Fig. 2.
Patterns of developmental expression of the glutamate receptor subunits GluR1, GluR2/3, NR1, NR2A/B, NR2A, and NR2B. P2, P10, P35, and 6 month (6M) rat hippocampus homogenates (20 μg of protein/lane) were analyzed by SDS-PAGE and immunoblotting with affinity-purified antibodies: GluR1 (9T), GluR2/3 (25-8), NR1 (clone 54.1), NR2A/B (T12), NR2A (T58), and NR2B (T51). At all ages, samples analyzed with the different antibodies were obtained from the same preparation of hippocampus. Histograms show the relative amount of protein (in percent P35). Levels were measured by densitometric scanning of Western blots.
Fig. 3.
Patterns of developmental expression of MAGUKs. P2, P10, P35, and 6 month (6M) rat hippocampus homogenates (20 μg of protein/lane) were analyzed by SDS-PAGE and immunoblotting with antibodies against PSD-93 (AL), SAP-102 (JH62514), and PSD-95 (TL). At all ages, samples analyzed with the different antibodies were obtained from the same preparation of hippocampus. Histograms show the relative amount of protein (in percent P35). Levels were measured by densitometric scanning of Western blots.
Fig. 4.
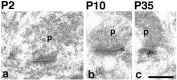
A, Immunogold labeling of PSD-95 in the CA1 stratum radiatum of the hippocampus. Postembedding immunogold labeling (10 nm) was performed using either of two PSD-95 antibodies (b–e, g, h, M, TL; a, f, R, JH), at P2 (a, b), P10 (c, d), P35 (e, f), and 6 months (g, h).p, Presynaptic terminal. Postsynaptic structures include dendrite shafts (b, synapse in a,top right) and spines (c–h); the synapse in the bottom left of a is at the enlarged base of a filopodium extending to the left (data not shown). Scale bar, 0.3 μm. Representative micrographs were chosen to illustrate the increase in PSD-95 labeling in synapses with age.B, Immunogold labeling of SAP-102 in the CA1 stratum radiatum of the hippocampus. Postembedding immunogold labeling (10 nm) was done using either of two SAP-102 antibodies (a, b, d–f, h, i, R, JH; c, g, GP-DB) at P2 (a–c), P10 (d, e), P35 (f, g), and 6 months (h, i). p, Presynaptic terminal. Postsynaptic structures include dendrite shafts (b), protuberances (a, c), and spines (d–i). Scale bar, 0.3 μm. Representative micrographs were chosen to illustrate the decrease in SAP-102 labeling in synapses with age. C, Summary of developmental changes in the number of gold particles per synapse and the number of gold particles per labeled synapse (see Table 2) at P2, P10, P35, and 6 months (6M). Error bars indicate SE.
Fig. 5.
Double labeling for SAP-102 (R, JH; 5 nm gold) and PSD-95 (M, TL; 15 nm gold) in the CA1 stratum radiatum of the hippocampus, at P2 (a, b), P10 (c, d), and P35 (e–j). Five nanometer gold in a, c, e, and g is indicated by_arrowheads_ and shown at a higher magnification in_b, d, f_, and h, respectively.p, Presynaptic terminal. Postsynaptic structures include dendrite shafts (a, b), protuberances (c, d), and spines (e–j). Scale bar: a, c, 0.3 μm; e, g, i, j, 0.2 μm; b, d, f, h, 0.1 μm. Representative micrographs were chosen to illustrate the decrease in SAP-102 and the increase in PSD-95 in synapses with age, and to show examples of single- and double-labeled synapses in adults.
Fig. 6.
Immunogold labeling of PSD-93 (R, AL) in the CA1 stratum radiatum of the hippocampus. Postembedding immunogold labeling (10 nm) was performed at P2 (a), P10 (b), and P35 (c).p, Presynaptic terminal. Postsynaptic structures include dendrite shafts (a, b) and spines (c). Scale bar, 0.3 μm. Representative micrographs were chosen to illustrate the increase in PSD-93 labeling in synapses with age, similar to that seen for PSD-95.
Fig. 7.
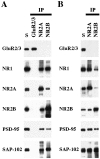
Co-immunoprecipitation of PSD-95 and SAP-102 with NR2 subunits. Whole hippocampus (A) or the CA1–CA2 region of the hippocampus (B) was solubilized in 1% DOC, and GluR2/3, NR2A, or NR2B were immunoprecipitated using 25-8 (GluR2/3) antibody, T58 NR2A serum, or T51 NR2B serum. Ten microliters of the total soluble protein (S) and 10 μl of the bound immunoprecipitate fractions were resolved by SDS-PAGE and probed with GluR2/3 (25-8), NR1 (clone 54.1), NR2A (2F6.3D5), NR2B (TL), PSD-95 (TL), and SAP-102 (JH62514). Because the immunoprecipitates were resuspended in 0.1 volume of the original soluble fraction, the bound sample is 10 times more concentrated than the total soluble fraction.
Fig. 8.
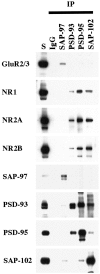
Co-immunoprecipitation of NR subunits with PSD-93, PSD-95, or SAP-102 in whole hippocampus. Hippocampi were solubilized in 1% DOC, and SAP-97, PSD-93, PSD-95, and SAP-102 were immunoprecipitated using JH62426, PSD-93 AL, T60, or JH62514 sera, respectively. Ten microliters of the total soluble protein input (S) and 10 μl of the bound immunoprecipitate fractions were separated by SDS-PAGE, immunoblotted, and incubated with antibodies against GluR2/3 (25-8), NR1 (clone 54.1), NR2A (2F6.3D5), NR2B (TL), SAP-97 (JH62426), PSD-93 (R-DB), PSD-95 (TL), or SAP-102 (JH62514). Because the immunoprecipitates were resuspended in 0.1 volume of the original soluble fraction, the bound sample is 10 times more concentrated than the total soluble fraction.
Fig. 9.
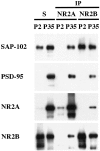
Comparison of co-immunoprecipitation of PSD-95 and SAP-102 with NR2 subunits at P2 and P35. P2 and P35 hippocampi were solubilized in 1% DOC, and the same amount of protein was used for immunoprecipitation of NR2A and NR2B using T58 NR2A or T51 NR2B sera, respectively. Total soluble protein (S) and the bound immunoprecipitate fractions were resolved by SDS-PAGE and probed with NR2A (2F6.3D5), NR2B (TL), PSD-95 (TL), and SAP-102 (JH62514).
Fig. 10.
Immunogold labeling (10 nm gold) of NR1 (a, b), NR2A/B (c–e), PSD-95 (f, g; M, TL), and SAP-102 (h–j; R, JH) in nonsynaptic surface specializations (arrowheads) in the CA1 stratum radiatum of the hippocampus at P2. Scale bar, 0.2 μm.
Similar articles
- Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex.
Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Aoki C, et al. Synapse. 2001 Jun 15;40(4):239-57. doi: 10.1002/syn.1047. Synapse. 2001. PMID: 11309840 - Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development.
Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Elias GM, et al. Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20953-8. doi: 10.1073/pnas.0811025106. Epub 2008 Dec 22. Proc Natl Acad Sci U S A. 2008. PMID: 19104036 Free PMC article. - Promiscuous interactions between AMPA-Rs and MAGUKs.
Fitzjohn SM, Doherty AJ, Collingridge GL. Fitzjohn SM, et al. Neuron. 2006 Oct 19;52(2):222-4. doi: 10.1016/j.neuron.2006.10.002. Neuron. 2006. PMID: 17046684 Review. - Trans-synaptic LGI1-ADAM22-MAGUK in AMPA and NMDA receptor regulation.
Fukata Y, Hirano Y, Miyazaki Y, Yokoi N, Fukata M. Fukata Y, et al. Neuropharmacology. 2021 Aug 15;194:108628. doi: 10.1016/j.neuropharm.2021.108628. Epub 2021 Jun 3. Neuropharmacology. 2021. PMID: 34089731 Review.
Cited by
- Key amino acid residues within the third membrane domains of NR1 and NR2 subunits contribute to the regulation of the surface delivery of N-methyl-D-aspartate receptors.
Kaniakova M, Krausova B, Vyklicky V, Korinek M, Lichnerova K, Vyklicky L, Horak M. Kaniakova M, et al. J Biol Chem. 2012 Jul 27;287(31):26423-34. doi: 10.1074/jbc.M112.339085. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711533 Free PMC article. - Short-term field stimulation mimics synaptic maturation of hippocampal synapses.
Bagley EE, Westbrook GL. Bagley EE, et al. J Physiol. 2012 Apr 1;590(7):1641-54. doi: 10.1113/jphysiol.2011.224964. Epub 2012 Feb 20. J Physiol. 2012. PMID: 22351628 Free PMC article. - Electrical Responses and Spontaneous Activity of Human iPS-Derived Neuronal Networks Characterized for 3-month Culture with 4096-Electrode Arrays.
Amin H, Maccione A, Marinaro F, Zordan S, Nieus T, Berdondini L. Amin H, et al. Front Neurosci. 2016 Mar 30;10:121. doi: 10.3389/fnins.2016.00121. eCollection 2016. Front Neurosci. 2016. PMID: 27065786 Free PMC article. - NMDA receptor-dependent long-term depression in the lateral habenula: implications in physiology and depression.
Kang M, Noh J, Chung JM. Kang M, et al. Sci Rep. 2020 Oct 21;10(1):17921. doi: 10.1038/s41598-020-74496-w. Sci Rep. 2020. PMID: 33087756 Free PMC article. - Organization of NMDA receptors at extrasynaptic locations.
Petralia RS, Wang YX, Hua F, Yi Z, Zhou A, Ge L, Stephenson FA, Wenthold RJ. Petralia RS, et al. Neuroscience. 2010 Apr 28;167(1):68-87. doi: 10.1016/j.neuroscience.2010.01.022. Epub 2010 Jan 20. Neuroscience. 2010. PMID: 20096331 Free PMC article.
References
- Bar-Peled M, Raikhel NV. A method for isolation and purification of specific antibodies to a protein fused to the GST. Anal Biochem. 1996;241:140–142. - PubMed
- Bassand P, Bernard A, Rafiki A, Gayet D, Khrestchatisky M. Differential interaction of the tSXV motifs of the NR1 and NR2A NMDA receptor subunits with PSD-95 and SAP-97. Eur J Neurosci. 1999;11:2031–2043. - PubMed
- Blahos IIJ, Wenthold RJ. Relationship between NMDA receptor NR1 splice variants and NR2 subunits. J Biol Chem. 1996;271:15669–15674. - PubMed
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and 1-syntrophin mediated by PDZ domains. Cell. 1996a;84:757–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous