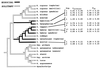High relatedness and inbreeding at the origin of eusociality in gall-inducing thrips - PubMed (original) (raw)
High relatedness and inbreeding at the origin of eusociality in gall-inducing thrips
T W Chapman et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Within the haplodiploid eusocial gall-inducing thrips, a species-level phylogeny combined with genetic data for five eusocial species enables an inference of levels of relatedness and inbreeding values for lineages at the origin of eusociality. Character optimization using data from five eusocial species indicates that the lineage or lineages where eusociality is inferred to have originated exhibit relatedness of 0.64-0.92, and F(IS) of 0.33-0.64. The high inbreeding coefficients found in these eusocial thrips have increased relatedness among and within both sexes and have reduced the haplodiploidy-induced relatedness asymmetries [Hamilton, W. D. (1964) J. Theor. Biol. 7, 1-52]. These results indicate that unusually high relatedness is associated with the origin of eusociality, and they suggest a role for inbreeding in the evolution of bisexual helping.
Figures
Figure 1
Maximum-parsimony bootstrap 50% majority-rule consensus tree (plus compatible groups) of solitary and eusocial thrips, from Crespi et al. (15). Bootstrap values >50%, from 500 replicates, appear beside branches. Two plausible scenarios for the origin of the soldier caste are inferred: (i) one origin with the sister taxa K. hamiltoni and K. harpophyllae basal to all eusocial taxa or (ii) two origins with Oncothrips morrisi as the most basal of the eusocial Oncothrips and K. hamiltoni and K. harpophyllae represent a second independent origin of the soldier caste. Female soldier inbreeding and among-soldier relatedness values (for both sexes combined) are given, with associated jacknife standard errors (12). Bracketed names are species names for Acacia host plants (6, 14). Estimates for Oncothrips tepperi population 2 are for disperser females rather than soldiers. We have been collecting these thrips throughout Australia for 10 years and are confident that additional species with soldiers, basal to K. hamiltoni and K. harpophyllae, are unlikely to exist. The genus name Onychothrips is abbreviated as “Ony.” An asterisk indicates that estimates were published elsewhere (10).
Similar articles
- Inbreeding ancestors: the role of sibmating in the social evolution of gall thrips.
McLeish MJ, Chapman TW, Crespi BJ. McLeish MJ, et al. J Hered. 2006 Jan-Feb;97(1):31-8. doi: 10.1093/jhered/esj001. Epub 2006 Jan 4. J Hered. 2006. PMID: 16394258 - Phylogenetics of social behavior in Australian gall-forming thrips: evidence from mitochondrial DNA sequence, adult morphology and behavior, and gall morphology.
Crespi BJ, Carmean DA, Mound LA, Worobey M, Morris D. Crespi BJ, et al. Mol Phylogenet Evol. 1998 Feb;9(1):163-80. doi: 10.1006/mpev.1997.0449. Mol Phylogenet Evol. 1998. PMID: 9479705 - Host-driven diversification of gall-inducing Acacia thrips and the aridification of Australia.
McLeish MJ, Chapman TW, Schwarz MP. McLeish MJ, et al. BMC Biol. 2007 Jan 26;5:3. doi: 10.1186/1741-7007-5-3. BMC Biol. 2007. PMID: 17257412 Free PMC article. - A conceptual model for the origin of worker behaviour and adaptation of eusociality.
Hunt JH. Hunt JH. J Evol Biol. 2012 Jan;25(1):1-19. doi: 10.1111/j.1420-9101.2011.02421.x. Epub 2011 Nov 23. J Evol Biol. 2012. PMID: 22111867 Review. - The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib-social effects, and heterochrony.
Linksvayer TA, Wade MJ. Linksvayer TA, et al. Q Rev Biol. 2005 Sep;80(3):317-36. doi: 10.1086/432266. Q Rev Biol. 2005. PMID: 16250466 Review.
Cited by
- Caste Transition and Reversion in Harpegnathos saltator Ant Colonies.
Opachaloemphan C, Carmona-Aldana F, Yan H. Opachaloemphan C, et al. Bio Protoc. 2023 Aug 20;13(16):e4770. doi: 10.21769/BioProtoc.4770. eCollection 2023 Aug 20. Bio Protoc. 2023. PMID: 37638295 Free PMC article. - The soldiers in societies: defense, regulation, and evolution.
Tian L, Zhou X. Tian L, et al. Int J Biol Sci. 2014 Mar 5;10(3):296-308. doi: 10.7150/ijbs.6847. eCollection 2014. Int J Biol Sci. 2014. PMID: 24644427 Free PMC article. Review. - The validity and value of inclusive fitness theory.
Bourke AF. Bourke AF. Proc Biol Sci. 2011 Nov 22;278(1723):3313-20. doi: 10.1098/rspb.2011.1465. Epub 2011 Sep 14. Proc Biol Sci. 2011. PMID: 21920980 Free PMC article. Review. - Individual responsiveness to shock and colony-level aggression in honey bees: evidence for a genetic component.
Avalos A, Rodríguez-Cruz Y, Giray T. Avalos A, et al. Behav Ecol Sociobiol. 2014 May;68(5):761-771. doi: 10.1007/s00265-014-1689-8. Behav Ecol Sociobiol. 2014. PMID: 25729126 Free PMC article. - Multilevel selection and social evolution of insect societies.
Korb J, Heinze J. Korb J, et al. Naturwissenschaften. 2004 Jun;91(6):291-304. doi: 10.1007/s00114-004-0529-5. Epub 2004 Apr 24. Naturwissenschaften. 2004. PMID: 15241605 Review.
References
- Mound L A. In: Plant Galls. Williams M A J, editor. Oxford: Oxford Univ. Press; 1994. pp. 131–149.
- Crespi B J. Nature (London) 1992;359:724–726.
- Crespi B J. J Nat Hist. 1992;26:769–809.
- Crespi B J, Mound L A. In: The Evolution of Social Behavior in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 166–180.
- Mound L A, Crespi B J, Kranz B. Invertebr Tax. 1996;10:1171–1198.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources