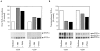The tetraspanin CD9 associates with transmembrane TGF-alpha and regulates TGF-alpha-induced EGF receptor activation and cell proliferation - PubMed (original) (raw)
The tetraspanin CD9 associates with transmembrane TGF-alpha and regulates TGF-alpha-induced EGF receptor activation and cell proliferation
W Shi et al. J Cell Biol. 2000.
Abstract
Transforming growth factor-alpha (TGF-alpha) is a member of the EGF growth factor family. Both transmembrane TGF-alpha and the proteolytically released soluble TGF-alpha can bind to the EGF/TGF-alpha tyrosine kinase receptor (EGFR) and activate the EGFR-induced signaling pathways. We now demonstrate that transmembrane TGF-alpha physically interacts with CD9, a protein with four membrane spanning domains that is frequently coexpressed with TGF-alpha in carcinomas. This interaction was mediated through the extracellular domain of transmembrane TGF-alpha. CD9 expression strongly decreased the growth factor- and PMA- induced proteolytic conversions of transmembrane to soluble TGF-alpha and strongly enhanced the TGF- alpha-induced EGFR activation, presumably in conjunction with increased expression of transmembrane TGF-alpha. In juxtacrine assays, the CD9-induced EGFR hyperactivation by transmembrane TGF-alpha resulted in increased proliferation. In contrast, CD9 coexpression with transmembrane TGF-alpha decreased the autocrine growth stimulatory effect of TGF-alpha in epithelial cells. This decrease was associated with increased expression of the cdk inhibitor, p21(CIP1). These data reveal that the association of CD9 with transmembrane TGF-alpha regulates ligand-induced activation of the EGFR, and results in altered cell proliferation.
Figures
Figure 1
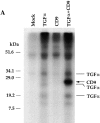
Association of CD9 with transmembrane TGF-α. (A) Anti–TGF-α immunoprecipitates of 35S-labeled, transfected 293 cell lysates were analyzed using SDS-PAGE. Cells were transfected with control pRK5 plasmid (lane 1), pRK7-TGF-α (lane 2), pRK7-CD9 (lane 3), and pRK7-TGF-α and pRK7-CD9 (lane 4). The previously characterized three transmembrane forms of TGF-α (Bringman et al. 1987) are marked. CD9 is marked with an arrow. As shown, CD9 coprecipitates with transmembrane TGF-α. (B) Anti–TGF-α immunoprecipitates of unlabeled, transfected 293 cells were analyzed by Western blotting using an anti–CD9 antibody. Cells were transfected with control pRK5 plasmid (lane 1) or with expression plasmids for TGF-α and/or CD9, as marked (lanes 2–4). CD9 is marked with an arrow, whereas nonspecific bands are not marked. (C) CD9 does not associate with the EGFR. Anti–TGF-α or anti-EGFR immunoprecipitates of unlabeled, transfected CHO cells were analyzed by Western blotting using an anti–CD9 antibody. Cells were transfected with expression plasmids for TGF-α and/or CD9, as marked. CD9 is marked with an arrow, whereas nonspecific bands are not marked.
Figure 1
Association of CD9 with transmembrane TGF-α. (A) Anti–TGF-α immunoprecipitates of 35S-labeled, transfected 293 cell lysates were analyzed using SDS-PAGE. Cells were transfected with control pRK5 plasmid (lane 1), pRK7-TGF-α (lane 2), pRK7-CD9 (lane 3), and pRK7-TGF-α and pRK7-CD9 (lane 4). The previously characterized three transmembrane forms of TGF-α (Bringman et al. 1987) are marked. CD9 is marked with an arrow. As shown, CD9 coprecipitates with transmembrane TGF-α. (B) Anti–TGF-α immunoprecipitates of unlabeled, transfected 293 cells were analyzed by Western blotting using an anti–CD9 antibody. Cells were transfected with control pRK5 plasmid (lane 1) or with expression plasmids for TGF-α and/or CD9, as marked (lanes 2–4). CD9 is marked with an arrow, whereas nonspecific bands are not marked. (C) CD9 does not associate with the EGFR. Anti–TGF-α or anti-EGFR immunoprecipitates of unlabeled, transfected CHO cells were analyzed by Western blotting using an anti–CD9 antibody. Cells were transfected with expression plasmids for TGF-α and/or CD9, as marked. CD9 is marked with an arrow, whereas nonspecific bands are not marked.
Figure 1
Association of CD9 with transmembrane TGF-α. (A) Anti–TGF-α immunoprecipitates of 35S-labeled, transfected 293 cell lysates were analyzed using SDS-PAGE. Cells were transfected with control pRK5 plasmid (lane 1), pRK7-TGF-α (lane 2), pRK7-CD9 (lane 3), and pRK7-TGF-α and pRK7-CD9 (lane 4). The previously characterized three transmembrane forms of TGF-α (Bringman et al. 1987) are marked. CD9 is marked with an arrow. As shown, CD9 coprecipitates with transmembrane TGF-α. (B) Anti–TGF-α immunoprecipitates of unlabeled, transfected 293 cells were analyzed by Western blotting using an anti–CD9 antibody. Cells were transfected with control pRK5 plasmid (lane 1) or with expression plasmids for TGF-α and/or CD9, as marked (lanes 2–4). CD9 is marked with an arrow, whereas nonspecific bands are not marked. (C) CD9 does not associate with the EGFR. Anti–TGF-α or anti-EGFR immunoprecipitates of unlabeled, transfected CHO cells were analyzed by Western blotting using an anti–CD9 antibody. Cells were transfected with expression plasmids for TGF-α and/or CD9, as marked. CD9 is marked with an arrow, whereas nonspecific bands are not marked.
Figure 2
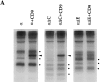
CD9 interacts with the extracellular domain of transmembrane TGF-α. (A) Interaction of CD9 with deletion mutants of transmembrane TGF-α. Anti–TGF-α immunoprecipitates of 35S-labeled, transfected 293 cell lysates were analyzed using SDS-PAGE. Cells were transfected with expression plasmids for transmembrane TGF-α (α), a cytoplasmically truncated transmembrane TGF-α (αΔC) or a transmembrane TGF-α mutant lacking the extracellular TGF-α core sequence (αΔE), with or without an expression plasmid for CD9. The arrowheads show CD9, which coprecipitates with transmembrane forms of wild-type TGF-α or TGF-αΔC but not with TGF-αΔE. The bullets show the three transmembrane TGF-α forms, which in the case of αΔC and αΔE are predictably smaller in size than in wild-type TGF-α. (B) Coexpression of HB-EGF competes with binding of transmembrane TGF-α to CD9. CHO cells were transfected with expression plasmids for TGF-α with or without CD9, in the presence or absence of transmembrane HB-EGF coexpression. Immunoprecipitation of transmembrane TGF-α resulted in coprecipitation of CD9, as shown in lane 3, and the level of coprecipitated CD9 was considerably less, when HB-EGF was coexpressed (lane 4). In this experiment, the expression of the three transmembrane TGF-α forms (marked with bullets) is considerably less than in Fig. 1 A.
Figure 2
CD9 interacts with the extracellular domain of transmembrane TGF-α. (A) Interaction of CD9 with deletion mutants of transmembrane TGF-α. Anti–TGF-α immunoprecipitates of 35S-labeled, transfected 293 cell lysates were analyzed using SDS-PAGE. Cells were transfected with expression plasmids for transmembrane TGF-α (α), a cytoplasmically truncated transmembrane TGF-α (αΔC) or a transmembrane TGF-α mutant lacking the extracellular TGF-α core sequence (αΔE), with or without an expression plasmid for CD9. The arrowheads show CD9, which coprecipitates with transmembrane forms of wild-type TGF-α or TGF-αΔC but not with TGF-αΔE. The bullets show the three transmembrane TGF-α forms, which in the case of αΔC and αΔE are predictably smaller in size than in wild-type TGF-α. (B) Coexpression of HB-EGF competes with binding of transmembrane TGF-α to CD9. CHO cells were transfected with expression plasmids for TGF-α with or without CD9, in the presence or absence of transmembrane HB-EGF coexpression. Immunoprecipitation of transmembrane TGF-α resulted in coprecipitation of CD9, as shown in lane 3, and the level of coprecipitated CD9 was considerably less, when HB-EGF was coexpressed (lane 4). In this experiment, the expression of the three transmembrane TGF-α forms (marked with bullets) is considerably less than in Fig. 1 A.
Figure 3
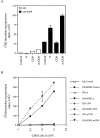
CD9 expression inhibits the serum- or PMA-induced proteolytic release of soluble TGF-α. (A) CD9 expression decreases the serum- or PMA-induced release of soluble TGF-α into the medium. The bottom panel shows the soluble TGF-α in the medium; the smaller form is the 50–amino acid form, whereas the larger one, which is much lighter in intensity, is the glycosylated form. The top panel shows the average values of duplicate samples, based on liquid scintillation counting of the immunoprecipitated TGF-α in the medium. The amounts of free TGF-α released into the medium were normalized to the 100% value for the unstimulated control samples (B) The serum- or PMA-induced processing of transmembrane TGF-α is decreased in the presence of CD9. Transmembrane TGF-α was immunoprecipitated from the lysates and visualized by SDS-PAGE and autoradiography (bottom). As shown, serum or PMA induced ectodomain cleavage and a resulting decrease of transmembrane TGF-α. CD9 inhibited this decrease in cell-associated TGF-α. The top panel shows a quantitation of the cell-associated TGF-α by phosphoimage analysis of the immunoprecipitated cell-associated TGF-α. All experiments were carried out in duplicate, and the values were averaged and normalized to the 100% value for the unstimulated control samples.
Figure 4
Increased EGFR tyrosine phosphorylation in response to transmembrane TGF-α/CD9 costimulation. (A) 32D cells or EGFR expressing 32D cells (32D-EGFR) were brought in contact with CHO cells expressing transmembrane TGF-α, CD9, or both, as shown. EGFR tyrosine phosphorylation in the 32D-EGFR cells was assessed by antiphosphotyrosine Western blotting reactivity of the 170-kD EGFR band using equal amounts of cell lysate protein. The autoradiographic intensity of the phosphorylated EGFR, induced by TGF-α/CD9 coexpressing cells (α/CD9), was 1.33-fold higher than the EGFR phosphorylation, induced by TGF-α–expressing cells (α), as assessed by scanning of the autoradiogram. (B) Tyrosine phosphorylation of endogenous EGFR in MDCK cells, stably transfected with expression plasmids for transmembrane TGF-α, CD9, or both. EGFR tyrosine phosphorylation was assessed as in A. Scanning revealed a 1.42-fold higher intensity of the phosphorylated EGFR band in TGF-α/CD9 coexpressing cells (α/CD9), when compared with the cells that only overexpress TGF-α (α).
Figure 4
Increased EGFR tyrosine phosphorylation in response to transmembrane TGF-α/CD9 costimulation. (A) 32D cells or EGFR expressing 32D cells (32D-EGFR) were brought in contact with CHO cells expressing transmembrane TGF-α, CD9, or both, as shown. EGFR tyrosine phosphorylation in the 32D-EGFR cells was assessed by antiphosphotyrosine Western blotting reactivity of the 170-kD EGFR band using equal amounts of cell lysate protein. The autoradiographic intensity of the phosphorylated EGFR, induced by TGF-α/CD9 coexpressing cells (α/CD9), was 1.33-fold higher than the EGFR phosphorylation, induced by TGF-α–expressing cells (α), as assessed by scanning of the autoradiogram. (B) Tyrosine phosphorylation of endogenous EGFR in MDCK cells, stably transfected with expression plasmids for transmembrane TGF-α, CD9, or both. EGFR tyrosine phosphorylation was assessed as in A. Scanning revealed a 1.42-fold higher intensity of the phosphorylated EGFR band in TGF-α/CD9 coexpressing cells (α/CD9), when compared with the cells that only overexpress TGF-α (α).
Figure 5
CD9 coexpression enhances the cell surface presentation of transmembrane TGF-α. (A) Cell surface expression of transmembrane TGF-α in CHO cells, stably transfected with expression plasmids for TGF-α, CD9, or both. Intact cells were incubated with the anti–TGF-α mAb α1, before lysis and anti–TGF-α immunoprecipitation, followed by anti–TGF-α Western blotting using Ab-1 antibody (Calbiochem-Novabiochem). The three forms of transmembrane TGF-α are marked. The nature of the sharp, unmarked band is not known, but may be due to an artifact in gel migration. Scanning of the upper and lower TGF-α band on the autoradiogram revealed a 1.68- and 1.24-fold higher intensity, respectively, in the TGF-α/CD9 coexpressing cells (α/CD9) when compared with the cells that only overexpress TGF-α (α). (B) CD9 coexpression results in enhanced levels of transmembrane TGF-α in MDCK clones. Besides the clone, marked α/CD9, which was used in further experiments, another clone (clone 2) also showed enhanced TGF-α levels when compared with the TGF-α overexpressing MDCK cells (marked α). Control marks the parental MDCK cells, which did not overexpress TGF-α or CD9, whereas CD9 designates MDCK cells that overexpress CD9 but not TGF-α. Transmembrane TGF-α was assessed by immunoprecipitation with anti–TGF-α antibody, followed by Western blot using anti–TGF-α antibody. The TGF-α form shown corresponds to the large glycosylated form. The nonspecific band was detectable in all lanes. (C) Immunostaining of transmembrane TGF-α in permeabilized MDCK cells, stably transfected with an expression plasmid for TGF-α in the absence (left) or presence (right) of overexpressed CD9. Immunoprecipitation of 35S-labeled cell lysates confirmed the higher expression of transmembrane TGF-α in TGF-α/CD9 coexpressing cells, which, based on scanning of the autoradiogram, was 4.4-fold higher than in the cells that only overexpress TGF-α (data not shown).
Figure 5
CD9 coexpression enhances the cell surface presentation of transmembrane TGF-α. (A) Cell surface expression of transmembrane TGF-α in CHO cells, stably transfected with expression plasmids for TGF-α, CD9, or both. Intact cells were incubated with the anti–TGF-α mAb α1, before lysis and anti–TGF-α immunoprecipitation, followed by anti–TGF-α Western blotting using Ab-1 antibody (Calbiochem-Novabiochem). The three forms of transmembrane TGF-α are marked. The nature of the sharp, unmarked band is not known, but may be due to an artifact in gel migration. Scanning of the upper and lower TGF-α band on the autoradiogram revealed a 1.68- and 1.24-fold higher intensity, respectively, in the TGF-α/CD9 coexpressing cells (α/CD9) when compared with the cells that only overexpress TGF-α (α). (B) CD9 coexpression results in enhanced levels of transmembrane TGF-α in MDCK clones. Besides the clone, marked α/CD9, which was used in further experiments, another clone (clone 2) also showed enhanced TGF-α levels when compared with the TGF-α overexpressing MDCK cells (marked α). Control marks the parental MDCK cells, which did not overexpress TGF-α or CD9, whereas CD9 designates MDCK cells that overexpress CD9 but not TGF-α. Transmembrane TGF-α was assessed by immunoprecipitation with anti–TGF-α antibody, followed by Western blot using anti–TGF-α antibody. The TGF-α form shown corresponds to the large glycosylated form. The nonspecific band was detectable in all lanes. (C) Immunostaining of transmembrane TGF-α in permeabilized MDCK cells, stably transfected with an expression plasmid for TGF-α in the absence (left) or presence (right) of overexpressed CD9. Immunoprecipitation of 35S-labeled cell lysates confirmed the higher expression of transmembrane TGF-α in TGF-α/CD9 coexpressing cells, which, based on scanning of the autoradiogram, was 4.4-fold higher than in the cells that only overexpress TGF-α (data not shown).
Figure 5
CD9 coexpression enhances the cell surface presentation of transmembrane TGF-α. (A) Cell surface expression of transmembrane TGF-α in CHO cells, stably transfected with expression plasmids for TGF-α, CD9, or both. Intact cells were incubated with the anti–TGF-α mAb α1, before lysis and anti–TGF-α immunoprecipitation, followed by anti–TGF-α Western blotting using Ab-1 antibody (Calbiochem-Novabiochem). The three forms of transmembrane TGF-α are marked. The nature of the sharp, unmarked band is not known, but may be due to an artifact in gel migration. Scanning of the upper and lower TGF-α band on the autoradiogram revealed a 1.68- and 1.24-fold higher intensity, respectively, in the TGF-α/CD9 coexpressing cells (α/CD9) when compared with the cells that only overexpress TGF-α (α). (B) CD9 coexpression results in enhanced levels of transmembrane TGF-α in MDCK clones. Besides the clone, marked α/CD9, which was used in further experiments, another clone (clone 2) also showed enhanced TGF-α levels when compared with the TGF-α overexpressing MDCK cells (marked α). Control marks the parental MDCK cells, which did not overexpress TGF-α or CD9, whereas CD9 designates MDCK cells that overexpress CD9 but not TGF-α. Transmembrane TGF-α was assessed by immunoprecipitation with anti–TGF-α antibody, followed by Western blot using anti–TGF-α antibody. The TGF-α form shown corresponds to the large glycosylated form. The nonspecific band was detectable in all lanes. (C) Immunostaining of transmembrane TGF-α in permeabilized MDCK cells, stably transfected with an expression plasmid for TGF-α in the absence (left) or presence (right) of overexpressed CD9. Immunoprecipitation of 35S-labeled cell lysates confirmed the higher expression of transmembrane TGF-α in TGF-α/CD9 coexpressing cells, which, based on scanning of the autoradiogram, was 4.4-fold higher than in the cells that only overexpress TGF-α (data not shown).
Figure 6
CD9 increases the juxtacrine transmembrane TGF-α–induced DNA synthesis. (A) 32D cells, either parental or EGFR-expressing cells, were laid on top of a confluent monolayer of live CHO cells expressing TGF-α, CD9, or both (as marked), and [3H]dT incorporation in the 32D cells was measured. The values, shown as bar graphs, represent the averages of triplicates in two independent experiments with their SEM. (B) Cell number–dependent, juxtacrine stimulation of DNA synthesis. The experiments were done as in A, except that the 32D cells were incubated with different numbers of fixed CHO cells, as shown in the abscissa. Only the EGFR expressing 32D cells were stimulated by TGF-α–expressing CHO cells, and this stimulation was higher when TGF-α was coexpressed with CD9. None of the other cell combinations showed stimulation. In several cases, the SEM values are too low to be visualized in the diagram.
Figure 7
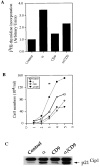
CD9 decreases the autocrine transmembrane TGF-α–induced DNA synthesis. (A) DNA synthesis rates in stably transfected MDCK cells. [3H]dT incorporation in transfected MDCK cells expressing TGF-α, CD9, or both, was measured as in Fig. 6. The values, shown as bar graphs, represent the averages of triplicates in two independent experiments with their SEM, which are too small to be seen in this experiment. (B) Cell proliferation, as measured by cell numbers, in stably transfected MDCK cells, overexpressing TGF-α, CD9, or both. The SEM values are too low to be visualized in the diagram. (C) Endogenous expression of p21CIP1 in transfected MDCK cells, expressing TGF-α, CD9, or both, as assessed by immunoprecipitation followed by Western blot analysis.
Similar articles
- Association of tetraspanin CD9 with transmembrane TGF{alpha} confers alterations in cell-surface presentation of TGF{alpha} and cytoskeletal organization.
Imhof I, Gasper WJ, Derynck R. Imhof I, et al. J Cell Sci. 2008 Jul 1;121(Pt 13):2265-74. doi: 10.1242/jcs.021717. Epub 2008 Jun 10. J Cell Sci. 2008. PMID: 18544636 - A variant epidermal growth factor receptor exhibits altered type alpha transforming growth factor binding and transmembrane signaling.
Moriai T, Kobrin MS, Hope C, Speck L, Korc M. Moriai T, et al. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10217-21. doi: 10.1073/pnas.91.21.10217. Proc Natl Acad Sci U S A. 1994. PMID: 7937865 Free PMC article. - The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor.
Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E. Higashiyama S, et al. J Cell Biol. 1995 Mar;128(5):929-38. doi: 10.1083/jcb.128.5.929. J Cell Biol. 1995. PMID: 7876316 Free PMC article. - CD9 antigen interacts with heparin-binding EGF-like growth factor through its heparin-binding domain.
Sakuma T, Higashiyama S, Hosoe S, Hayashi S, Taniguchi N. Sakuma T, et al. J Biochem. 1997 Aug;122(2):474-80. doi: 10.1093/oxfordjournals.jbchem.a021776. J Biochem. 1997. PMID: 9378729 - Transforming growth factor alpha: expression, regulation and biological action of its integral membrane precursor.
Luetteke NC, Lee DC. Luetteke NC, et al. Semin Cancer Biol. 1990 Aug;1(4):265-75. Semin Cancer Biol. 1990. PMID: 2103501 Review.
Cited by
- The role of Tetraspanins in digestive system tumor development: update and emerging evidence.
Shao S, Bu Z, Xiang J, Liu J, Tan R, Sun H, Hu Y, Wang Y. Shao S, et al. Front Cell Dev Biol. 2024 Feb 8;12:1343894. doi: 10.3389/fcell.2024.1343894. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38389703 Free PMC article. Review. - The versatile roles of testrapanins in cancer from intracellular signaling to cell-cell communication: cell membrane proteins without ligands.
Zhou Z, Yang Z, Zhou L, Yang M, He S. Zhou Z, et al. Cell Biosci. 2023 Mar 20;13(1):59. doi: 10.1186/s13578-023-00995-8. Cell Biosci. 2023. PMID: 36941633 Free PMC article. Review. - Targeting receptor tyrosine kinases in ovarian cancer: Genomic dysregulation, clinical evaluation of inhibitors, and potential for combinatorial therapies.
Wei Y, Erfani S, Schweer D, de Gouvea R, Qadir J, Shi J, Cheng K, Wu D, Craven R, Wu Y, Olivier T, Baldwin LA, Zhou B, Zhou Y, Zhao W, Yang BB, Ueland FR, Yang XH. Wei Y, et al. Mol Ther Oncolytics. 2023 Feb 19;28:293-306. doi: 10.1016/j.omto.2023.02.006. eCollection 2023 Mar 16. Mol Ther Oncolytics. 2023. PMID: 36911068 Free PMC article. Review. - Tetraspanin CD9: A friend or foe of head and neck cancer (Review).
P C S, Shetty SS, Nalilu SK, Shetty PK, Patil P. P C S, et al. Oncol Rep. 2022 May;47(5):88. doi: 10.3892/or.2022.8299. Epub 2022 Mar 10. Oncol Rep. 2022. PMID: 35266009 Free PMC article. Review. - Adaptation of the Golgi Apparatus in Cancer Cell Invasion and Metastasis.
Bui S, Mejia I, Díaz B, Wang Y. Bui S, et al. Front Cell Dev Biol. 2021 Dec 10;9:806482. doi: 10.3389/fcell.2021.806482. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957124 Free PMC article. Review.
References
- Adachi M., Taki T., Konishi T., Huang C.I., Higashiyama M., Miyake M. Novel staging protocol for non-small-cell lung cancers according to MRP-1/CD9 and KAI1/CD82 gene expression. J. Clin. Oncol. 1998;16:1397–1406. - PubMed
- Alroy I., Yarden Y. The ErbB signaling network in embryogenesis and oncogenesissignal diversification through combinatorial ligand-receptor interactions. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1997;410:83–86. - PubMed
- Arribas J., Coodly L., Vollmer P., Kishimoto T.K., Rose-John S., Massagué J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J. Biol. Chem. 1996;271:11376–11382. - PubMed
- Ausubel, F.M., R. Brent, R.E. Kinston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1997. Current Protocols in Molecular Biology. Vol. 1. V.B. Chanda, editor. John Wiley & Sons, Inc.
- Baselga J., Mendelsohn J., Kim Y.M., Pandiella A. Autocrine regulation of membrane transforming growth factor-α cleavage. J. Biol. Chem. 1996;271:3279–3284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous