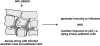The induction of tolerance by dendritic cells that have captured apoptotic cells - PubMed (original) (raw)
Comment
The induction of tolerance by dendritic cells that have captured apoptotic cells
R M Steinman et al. J Exp Med. 2000.
No abstract available
Figures
Figure 1
An illustration of the self-nonself problem from the perspective of DCs. During influenza infection of the airway, a site rich in DCs (reference 59), there is extensive apoptotic death of infected airway epithelial cells, which the DCs would likely process simultaneously with the influenza virus (references 29, 30).
Figure 3
Pathways whereby DCs might induce peripheral tolerance to antigens within tissue cells undergoing normal cell turnover by apoptosis. DC precursors traffic through tissues, phagocytose dying cells or apoptotic bodies, and then enter the lymph. Upon reaching the lymph node, the DCs tolerize naive, self-reactive T cells either directly, or indirectly (as diagrammed here) after reprocessing by different regulatory or tolerogenic DCs in the lymph node.
Figure 2
Diagram of the model of Kurts et al. (references 34, 35). Self-peptides in tissue cells, here the insulin-producing β cells of the pancreatic islets, are presented in a tolerogenic way by bone marrow–derived cells in the draining pancreatic lymph nodes.
Comment on
- Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells.
Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Sauter B, et al. J Exp Med. 2000 Feb 7;191(3):423-34. doi: 10.1084/jem.191.3.423. J Exp Med. 2000. PMID: 10662788 Free PMC article. - A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes.
Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. Huang FP, et al. J Exp Med. 2000 Feb 7;191(3):435-44. doi: 10.1084/jem.191.3.435. J Exp Med. 2000. PMID: 10662789 Free PMC article.
Similar articles
- Dendritic cells "in vivo": their role in the initiation of intestinal immune responses.
Liu L, MacPherson GG. Liu L, et al. Adv Exp Med Biol. 1995;371A:271-4. doi: 10.1007/978-1-4615-1941-6_56. Adv Exp Med Biol. 1995. PMID: 8525923 Review. No abstract available. - T cell receptor/MHC interactions in the thymus and the shaping of the T cell repertoire.
McDuffie M, Roehm N, Born W, Marrack P, Kappler JW. McDuffie M, et al. Transplant Proc. 1987 Dec;19(6 Suppl 7):111-6. Transplant Proc. 1987. PMID: 3321638 No abstract available. - Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen.
Zal T, Volkmann A, Stockinger B. Zal T, et al. J Exp Med. 1994 Dec 1;180(6):2089-99. doi: 10.1084/jem.180.6.2089. J Exp Med. 1994. PMID: 7964486 Free PMC article. - Mechanisms of Tolerance Induction by Dendritic Cells In Vivo.
Hasegawa H, Matsumoto T. Hasegawa H, et al. Front Immunol. 2018 Feb 26;9:350. doi: 10.3389/fimmu.2018.00350. eCollection 2018. Front Immunol. 2018. PMID: 29535726 Free PMC article. Review. - Evidence for involvement of clonal anergy in MHC class I and class II disparate skin allograft tolerance after the termination of intrathymic clonal deletion.
Tomita Y, Nishimura Y, Harada N, Eto M, Ayukawa K, Yoshikai Y, Nomoto K. Tomita Y, et al. J Immunol. 1990 Dec 15;145(12):4026-36. J Immunol. 1990. PMID: 2147933
Cited by
- Leishmania infantum and Leishmania braziliensis: Differences and Similarities to Evade the Innate Immune System.
Falcão Sde A, Jaramillo TM, Ferreira LG, Bernardes DM, Santana JM, Favali CB. Falcão Sde A, et al. Front Immunol. 2016 Aug 3;7:287. doi: 10.3389/fimmu.2016.00287. eCollection 2016. Front Immunol. 2016. PMID: 27536300 Free PMC article. - Annexin A1 on the surface of early apoptotic cells suppresses CD8+ T cell immunity.
Weyd H, Abeler-Dörner L, Linke B, Mahr A, Jahndel V, Pfrang S, Schnölzer M, Falk CS, Krammer PH. Weyd H, et al. PLoS One. 2013 Apr 30;8(4):e62449. doi: 10.1371/journal.pone.0062449. Print 2013. PLoS One. 2013. PMID: 23638088 Free PMC article. - The role of the vascular dendritic cell network in atherosclerosis.
Alberts-Grill N, Denning TL, Rezvan A, Jo H. Alberts-Grill N, et al. Am J Physiol Cell Physiol. 2013 Jul 1;305(1):C1-21. doi: 10.1152/ajpcell.00017.2013. Epub 2013 Apr 3. Am J Physiol Cell Physiol. 2013. PMID: 23552284 Free PMC article. Review. - Therapeutic potential of semi-mature dendritic cells for tolerance induction.
Lutz MB. Lutz MB. Front Immunol. 2012 May 18;3:123. doi: 10.3389/fimmu.2012.00123. eCollection 2012. Front Immunol. 2012. PMID: 22629255 Free PMC article. - Innate immunity and resistance to tolerogenesis in allotransplantation.
Benichou G, Tonsho M, Tocco G, Nadazdin O, Madsen JC. Benichou G, et al. Front Immunol. 2012 Apr 19;3:73. doi: 10.3389/fimmu.2012.00073. eCollection 2012. Front Immunol. 2012. PMID: 22566954 Free PMC article.
References
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A.M., Fathman C.G., Inaba K., Steinman R.M. Presentation of exogenous protein antigens by dendritic cells to T cell clonesintact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 1989;169:1169–1178. - PMC - PubMed
- Stumbles P.A., Thomas J.A., Pimm C.L., Lee P.T., Venaille T.J., Proksch S., Holt P.G. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 1998;188:2019–2031. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources