Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells - PubMed (original) (raw)
Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells
B Sauter et al. J Exp Med. 2000.
Abstract
Cell death by necrosis is typically associated with inflammation, in contrast to apoptosis. We have identified additional distinctions between the two types of death that occur at the level of dendritic cells (DCs) and which influence the induction of immunity. DCs must undergo changes termed maturation to act as potent antigen-presenting cells. Here, we investigated whether exposure to apoptotic or necrotic cells affected DC maturation. We found that immature DCs efficiently phagocytose a variety of apoptotic and necrotic tumor cells. However, only exposure to the latter induces maturation. The mature DCs express high levels of the DC-restricted markers CD83 and lysosome-associated membrane glycoprotein (DC-LAMP) and the costimulatory molecules CD40 and CD86. Furthermore, they develop into powerful stimulators of both CD4(+) and CD8(+) T cells. Cross-presentation of antigens to CD8(+) T cells occurs after uptake of apoptotic cells. We demonstrate here that optimal cross-presentation of antigens from tumor cells requires two steps: phagocytosis of apoptotic cells by immature DCs, which provides antigenic peptides for major histocompatibility complex class I and class II presentation, and a maturation signal that is delivered by exposure to necrotic tumor cells, their supernatants, or standard maturation stimuli, e.g., monocyte-conditioned medium. Thus, DCs are able to distinguish two types of tumor cell death, with necrosis providing a control that is critical for the initiation of immunity.
Figures
Figure 1
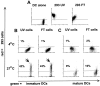
DCs phagocytose apoptotic cells and necrotic cells. 293 cells were labeled red with the PKH26GL fluorescent cell linker and induced to undergo apoptosis via UVB irradiation, or necrosis via repeated freezing and thawing. Immature DCs were dyed green with PKH67GL and then cocultured with the apoptotic and necrotic cells for 3 h at 4°C or 37°C at a ratio of 1:1. Cells were analyzed by FACScan™ where double positive cells indicate uptake of the apoptotic and necrotic cells by the DCs. Dot plots are gated on the FL1 high positive cells (DCs), thus excluding the dead 293 cells from the analysis. (A) Green-dyed DCs and red-dyed apoptotic (293 UV) and necrotic (293 FT) cells alone. (B) Uptake of apoptotic and necrotic cells by immature DCs. (C) Uptake of apoptotic and necrotic cells by mature DCs. The results shown are representative of 10 different experiments. (D and E) On day 6 of culture, 7 × 105 immature DCs were cultured with 5 × 106 green fluorescent microspheres in the absence (D) or presence (E) of MCM. On day 8, the DCs were separated from unengulfed beads by density gradient centrifugation and analyzed by FACScan™ for uptake of latex beads and CD83 expression.
Figure 1
DCs phagocytose apoptotic cells and necrotic cells. 293 cells were labeled red with the PKH26GL fluorescent cell linker and induced to undergo apoptosis via UVB irradiation, or necrosis via repeated freezing and thawing. Immature DCs were dyed green with PKH67GL and then cocultured with the apoptotic and necrotic cells for 3 h at 4°C or 37°C at a ratio of 1:1. Cells were analyzed by FACScan™ where double positive cells indicate uptake of the apoptotic and necrotic cells by the DCs. Dot plots are gated on the FL1 high positive cells (DCs), thus excluding the dead 293 cells from the analysis. (A) Green-dyed DCs and red-dyed apoptotic (293 UV) and necrotic (293 FT) cells alone. (B) Uptake of apoptotic and necrotic cells by immature DCs. (C) Uptake of apoptotic and necrotic cells by mature DCs. The results shown are representative of 10 different experiments. (D and E) On day 6 of culture, 7 × 105 immature DCs were cultured with 5 × 106 green fluorescent microspheres in the absence (D) or presence (E) of MCM. On day 8, the DCs were separated from unengulfed beads by density gradient centrifugation and analyzed by FACScan™ for uptake of latex beads and CD83 expression.
Figure 2
DCs exhibit a mature phenotype after exposure to necrotic but not apoptotic cell lines. (A) Apoptotic or necrotic B-LCL cells were cocultured with DCs at ratios of 1:2 or 1:5. After 48 h, the DCs were stained extracellularly for CD83 and intracellularly for CD83 and DC-LAMP. (B and C) DCs were cocultured with apoptotic or necrotic cell lines at a ratio of 1:2 for 48 h and then stained extracellularly for CD83 and intracellularly for DC-LAMP. (D) Immature and mature DCs were stained with mAbs to CD86, CD40, HLA-DR, and/or CD83. Apoptotic (Apop) or necrotic (F/T) cell lines (E), influenza-infected or uninfected macrophages (MO) (F), or supernatants from dead cells (G) were added to immature DCs. After 48 h, the DC were stained with mAbs to CD83, CD86, CD40, and HLA-DR. (H) Apoptotic and necrotic primary cells were cocultured for 48 h with DCs, after which the CD83 expression of the DCs was compared with DCs matured with MCM, LPS, or necrotic (F/T, or by hypotonic lysis) 293 cells. (I) Supernatants of apoptotic cells cultured for 8 and for 24 h or supernatants of partly ablated (1× F/T) or completely ablated (4× F/T) cells were added to immature DCs, and after 48 h the DCs were stained with an mAb to CD83. In D, E, and G, values represent the averages of the geometric mean indices from three or more experiments. Error bars mark the SD. In I, a directly labeled anti-CD83 (CD83-PE) antibody was used, accounting for the lower mean fluorescence intensities. Isotype-matched antibodies served as controls in all experiments (data not shown). In F, H, and I, a representative experiment of two to four is shown.
Figure 2
DCs exhibit a mature phenotype after exposure to necrotic but not apoptotic cell lines. (A) Apoptotic or necrotic B-LCL cells were cocultured with DCs at ratios of 1:2 or 1:5. After 48 h, the DCs were stained extracellularly for CD83 and intracellularly for CD83 and DC-LAMP. (B and C) DCs were cocultured with apoptotic or necrotic cell lines at a ratio of 1:2 for 48 h and then stained extracellularly for CD83 and intracellularly for DC-LAMP. (D) Immature and mature DCs were stained with mAbs to CD86, CD40, HLA-DR, and/or CD83. Apoptotic (Apop) or necrotic (F/T) cell lines (E), influenza-infected or uninfected macrophages (MO) (F), or supernatants from dead cells (G) were added to immature DCs. After 48 h, the DC were stained with mAbs to CD83, CD86, CD40, and HLA-DR. (H) Apoptotic and necrotic primary cells were cocultured for 48 h with DCs, after which the CD83 expression of the DCs was compared with DCs matured with MCM, LPS, or necrotic (F/T, or by hypotonic lysis) 293 cells. (I) Supernatants of apoptotic cells cultured for 8 and for 24 h or supernatants of partly ablated (1× F/T) or completely ablated (4× F/T) cells were added to immature DCs, and after 48 h the DCs were stained with an mAb to CD83. In D, E, and G, values represent the averages of the geometric mean indices from three or more experiments. Error bars mark the SD. In I, a directly labeled anti-CD83 (CD83-PE) antibody was used, accounting for the lower mean fluorescence intensities. Isotype-matched antibodies served as controls in all experiments (data not shown). In F, H, and I, a representative experiment of two to four is shown.
Figure 2
DCs exhibit a mature phenotype after exposure to necrotic but not apoptotic cell lines. (A) Apoptotic or necrotic B-LCL cells were cocultured with DCs at ratios of 1:2 or 1:5. After 48 h, the DCs were stained extracellularly for CD83 and intracellularly for CD83 and DC-LAMP. (B and C) DCs were cocultured with apoptotic or necrotic cell lines at a ratio of 1:2 for 48 h and then stained extracellularly for CD83 and intracellularly for DC-LAMP. (D) Immature and mature DCs were stained with mAbs to CD86, CD40, HLA-DR, and/or CD83. Apoptotic (Apop) or necrotic (F/T) cell lines (E), influenza-infected or uninfected macrophages (MO) (F), or supernatants from dead cells (G) were added to immature DCs. After 48 h, the DC were stained with mAbs to CD83, CD86, CD40, and HLA-DR. (H) Apoptotic and necrotic primary cells were cocultured for 48 h with DCs, after which the CD83 expression of the DCs was compared with DCs matured with MCM, LPS, or necrotic (F/T, or by hypotonic lysis) 293 cells. (I) Supernatants of apoptotic cells cultured for 8 and for 24 h or supernatants of partly ablated (1× F/T) or completely ablated (4× F/T) cells were added to immature DCs, and after 48 h the DCs were stained with an mAb to CD83. In D, E, and G, values represent the averages of the geometric mean indices from three or more experiments. Error bars mark the SD. In I, a directly labeled anti-CD83 (CD83-PE) antibody was used, accounting for the lower mean fluorescence intensities. Isotype-matched antibodies served as controls in all experiments (data not shown). In F, H, and I, a representative experiment of two to four is shown.
Figure 3
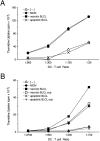
DCs gain heightened T cell stimulatory capacity after exposure to necrotic but not apoptotic cell lines. Apoptotic or necrotic cell lines (B-LCL) were added at a ratio of 1:5 to day 5 or 6 immature DCs. After 48 h of coculture, the DCs were assayed for their T cell stimulatory capacity. (A) DCs were irradiated with 3,000 rad using a cesium irradiator before addition to 2 × 105 syngeneic T cells and 0.1 ng/ml SEA. After 3 d, 4 μCi/ml [3H]thymidine was added for 16 h. (B) After coculture with apoptotic or necrotic cell lines (B-LCL) or their respective supernatants (sup), DCs were fixed with 1% paraformaldehyde for 30 min at 4°C, washed extensively, and added in graded doses to 2 × 105 allogeneic T cells. After 4 d, 4 μCi/ml [3H]thymidine was added for 16 h. Immature and MCM-matured DCs served as controls. T cells cultured alone gave counts <600 cpm. Results are representative of three or more experiments, and the values shown represent the mean of triplicate wells. (C) 5 × 105 DCs were cocultured with apoptotic B-LCL cells (ApopLCL) at a 2:1 ratio with or without the addition of 1 ng/ml LPS. After 16 h, the supernatants were tested for TNF-α release by ELISA.
Figure 3
DCs gain heightened T cell stimulatory capacity after exposure to necrotic but not apoptotic cell lines. Apoptotic or necrotic cell lines (B-LCL) were added at a ratio of 1:5 to day 5 or 6 immature DCs. After 48 h of coculture, the DCs were assayed for their T cell stimulatory capacity. (A) DCs were irradiated with 3,000 rad using a cesium irradiator before addition to 2 × 105 syngeneic T cells and 0.1 ng/ml SEA. After 3 d, 4 μCi/ml [3H]thymidine was added for 16 h. (B) After coculture with apoptotic or necrotic cell lines (B-LCL) or their respective supernatants (sup), DCs were fixed with 1% paraformaldehyde for 30 min at 4°C, washed extensively, and added in graded doses to 2 × 105 allogeneic T cells. After 4 d, 4 μCi/ml [3H]thymidine was added for 16 h. Immature and MCM-matured DCs served as controls. T cells cultured alone gave counts <600 cpm. Results are representative of three or more experiments, and the values shown represent the mean of triplicate wells. (C) 5 × 105 DCs were cocultured with apoptotic B-LCL cells (ApopLCL) at a 2:1 ratio with or without the addition of 1 ng/ml LPS. After 16 h, the supernatants were tested for TNF-α release by ELISA.
Figure 4
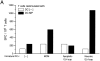
Immature DCs matured via necrotic supernatants are able to induce antigen-specific CD8+ effector cells. Cocultures of immature DCs were supplemented with nothing, MCM, or supernatants (sup) of apoptotic (UVB-irradiated B-LCL) or necrotic (frozen–thawed B-LCL) cells and incubated for 48 h. They were collected, unpulsed or pulsed with 1 μg/ml influenza matrix peptide for 1 h, and cocultured with 2 × 105 syngeneic T cells at a ratio of 1:30 for 7 d to allow for the activation of CD8+ effector cells. Responding T cells were collected and restimulated with autologous DCs that were untreated or pulsed with matrix peptide (MP) for 18 h. The induction of influenza matrix peptide–specific CD8+ T cells was measured in an IFN-γ ELISPOT assay (A). Alternatively, 7-d cocultures were directly tested for matrix peptide–specific CTL activity in a 4-h chromium-release assay using matrix peptide–pulsed T2 cells as targets (B). Results are representative of three experiments, and the values shown represent the mean of triplicate wells.
Figure 4
Immature DCs matured via necrotic supernatants are able to induce antigen-specific CD8+ effector cells. Cocultures of immature DCs were supplemented with nothing, MCM, or supernatants (sup) of apoptotic (UVB-irradiated B-LCL) or necrotic (frozen–thawed B-LCL) cells and incubated for 48 h. They were collected, unpulsed or pulsed with 1 μg/ml influenza matrix peptide for 1 h, and cocultured with 2 × 105 syngeneic T cells at a ratio of 1:30 for 7 d to allow for the activation of CD8+ effector cells. Responding T cells were collected and restimulated with autologous DCs that were untreated or pulsed with matrix peptide (MP) for 18 h. The induction of influenza matrix peptide–specific CD8+ T cells was measured in an IFN-γ ELISPOT assay (A). Alternatively, 7-d cocultures were directly tested for matrix peptide–specific CTL activity in a 4-h chromium-release assay using matrix peptide–pulsed T2 cells as targets (B). Results are representative of three experiments, and the values shown represent the mean of triplicate wells.
Figure 5
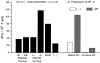
Exposure of immature DCs to maturation stimuli is necessary for optimal cross-presentation of antigen derived from apoptotic cells. EL-4 cells were infected or uninfected with influenza virus and induced to undergo apoptosis by UVB irradiation. Apoptotic cells were fed to immature DCs at a ratio of 1:20 and simultaneously exposed to MCM or supernatants (sup) from EL-4 cell lines. Supernatants were derived from UVB-irradiated EL-4 lines that were cultured for 8 vs. 24 h, or lines that had undergone one (1×) vs. four (4×) freeze–thaw cycles. After 48 h, the DCs were collected and cocultured with syngeneic T cells. Cross-presentation of influenza antigen was detected by monitoring IFN-γ–producing SFCs in an ELISPOT assay. The data shown were calculated after subtracting the background values, i.e., the number of SFCs obtained when apoptotic uninfected ELA cells were fed to immature DCs. These values ranged from 0 to 30/106 T cells. Additional controls consisted of immature and MCM-matured DCs, which were pulsed with 1 μg/ml of the influenza matrix peptide (MP) before coculture with syngeneic T cells.
Comment in
- The induction of tolerance by dendritic cells that have captured apoptotic cells.
Steinman RM, Turley S, Mellman I, Inaba K. Steinman RM, et al. J Exp Med. 2000 Feb 7;191(3):411-6. doi: 10.1084/jem.191.3.411. J Exp Med. 2000. PMID: 10662786 Free PMC article. No abstract available.
Similar articles
- Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells.
Albert ML, Jegathesan M, Darnell RB. Albert ML, et al. Nat Immunol. 2001 Nov;2(11):1010-7. doi: 10.1038/ni722. Nat Immunol. 2001. PMID: 11590405 - Monocyte-derived dendritic cells loaded with a mixture of apoptotic/necrotic melanoma cells efficiently cross-present gp100 and MART-1 antigens to specific CD8(+) T lymphocytes.
von Euw EM, Barrio MM, Furman D, Bianchini M, Levy EM, Yee C, Li Y, Wainstok R, Mordoh J. von Euw EM, et al. J Transl Med. 2007 Apr 20;5:19. doi: 10.1186/1479-5876-5-19. J Transl Med. 2007. PMID: 17448240 Free PMC article. - Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells.
Chen Z, Moyana T, Saxena A, Warrington R, Jia Z, Xiang J. Chen Z, et al. Int J Cancer. 2001 Aug 15;93(4):539-48. doi: 10.1002/ijc.1365. Int J Cancer. 2001. PMID: 11477558 - Sequential delivery of maturation stimuli increases human dendritic cell IL-12 production and enhances tumor antigen-specific immunogenicity.
Kalady MF, Onaitis MW, Emani S, Abdel-Wahab Z, Tyler DS, Pruitt SK. Kalady MF, et al. J Surg Res. 2004 Jan;116(1):24-31. doi: 10.1016/j.jss.2003.09.003. J Surg Res. 2004. PMID: 14732346 - Dendritic cell gene therapy.
Onaitis M, Kalady MF, Pruitt S, Tyler DS. Onaitis M, et al. Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
Cited by
- The mechanisms of action of vaccines containing aluminum adjuvants: an in vitro vs in vivo paradigm.
Ghimire TR. Ghimire TR. Springerplus. 2015 Apr 16;4:181. doi: 10.1186/s40064-015-0972-0. eCollection 2015. Springerplus. 2015. PMID: 25932368 Free PMC article. - NKG2D blockade inhibits poly(I:C)-triggered fetal loss in wild type but not in IL-10-/- mice.
Thaxton JE, Nevers T, Lippe EO, Blois SM, Saito S, Sharma S. Thaxton JE, et al. J Immunol. 2013 Apr 1;190(7):3639-47. doi: 10.4049/jimmunol.1203488. Epub 2013 Mar 1. J Immunol. 2013. PMID: 23455498 Free PMC article. - Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer.
Hannan R, Zhang H, Wallecha A, Singh R, Liu L, Cohen P, Alfieri A, Rothman J, Guha C. Hannan R, et al. Cancer Immunol Immunother. 2012 Dec;61(12):2227-38. doi: 10.1007/s00262-012-1257-x. Epub 2012 May 27. Cancer Immunol Immunother. 2012. PMID: 22644735 Free PMC article. - Multiple sclerosis: molecular mechanisms and therapeutic opportunities.
Miljković D, Spasojević I. Miljković D, et al. Antioxid Redox Signal. 2013 Dec 20;19(18):2286-334. doi: 10.1089/ars.2012.5068. Epub 2013 Apr 22. Antioxid Redox Signal. 2013. PMID: 23473637 Free PMC article. Review. - Up-Regulation of Interleukin-10 in Splenic Immune Response Induced by Serotype A Pasteurella multocida.
Li H, He M, Cheng Y, Jiang J, Yang W, Zhang Z, An Q, Chen S, Man C, Du L, Wang F, Chen Q. Li H, et al. Genes (Basel). 2022 Sep 3;13(9):1586. doi: 10.3390/genes13091586. Genes (Basel). 2022. PMID: 36140754 Free PMC article.
References
- Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br. Med. Bull. 1997;53:491–508 . - PubMed
- Cohen J.J. Overviewmechanisms of apoptosis. Immunol. Today. 1993;14:126–130. - PubMed
- Duvall E., Wyllie A.H. Death and the cell. Immunol. Today. 1986;7:115–119. - PubMed
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI044628/AI/NIAID NIH HHS/United States
- AI44628/AI/NIAID NIH HHS/United States
- GM07793/GM/NIGMS NIH HHS/United States
- AI39516/AI/NIAID NIH HHS/United States
- R37 AI044628/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials