Implications of all-or-none synaptic transmission and short-term depression beyond vesicle depletion: a computational study - PubMed (original) (raw)
Implications of all-or-none synaptic transmission and short-term depression beyond vesicle depletion: a computational study
V Matveev et al. J Neurosci. 2000.
Abstract
The all-or-none character of transmission at central synapses is commonly viewed as evidence that only one vesicle can be released per action potential at a single release site. This interpretation is still a matter of debate; its resolution is important for our understanding of the nature of quantal response. In this work we explore observable consequences of the univesicular release hypothesis by studying a stochastic model of synaptic transmission. We investigated several alternative mechanisms for the all-or-none response: (1) the univesicular release constraint realized through lateral inhibition across presynaptic membrane, (2) the constraint of a single releasable vesicle per active zone, and (3) the postsynaptic receptor saturation. We show that both the univesicular release constraint and the postsynaptic receptor saturation lead to a limited amount of depression by vesicle depletion, so that depletion alone cannot account for the strong paired-pulse depression observed at some cortical synapses. Although depression can be rapid if there is only one releasable vesicle per active zone, this scenario leads to a limit on the transmission probability. We evaluate additional mechanisms beyond vesicle depletion, and our results suggest that the strong paired-pulse depression may be a result of activity-dependent inactivation of the exocytosis machinery. Furthermore, we found that the statistical analysis of release events, in response to a long stimulus train, might allow one to distinguish experimentally between univesicular and multivesicular release scenarios. We show that without the univesicular release constraint, the temporal correlation between release events is always negative, whereas it is typically positive with such a constraint if the vesicle fusion probability is sufficiently large.
Figures
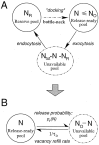
Fig. 1.
Models of vesicle turn-over. A, The two-pool synapse model. The “docked” pool is composed of vesicles immediately available for release. In response to an action potential, a docked vesicle is released with a certain probability dependent on ND. This pool is refilled by vesicles from the reserve pool; the dashed arrow signifies the bottleneck in the refill process. B, Single-pool synapse model. Release probability is described by a Poisson process with lateral inhibition between release sites and is given by 1 minus the failure rate, which is equal to exp(−αVN), where αVis the fusion rate for a single vesicle. Vacancy in the vesicle pool is refilled with a time constant of τD, which determines the depression recovery dynamics.
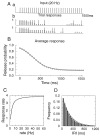
Fig. 2.
Response of the synapse model to constant frequency stimulation. A, An example with a stimulus rate of 20 Hz; (a) the stimulus train, (b) synaptic response; two sample trials are shown. _Black vertical bars_represent release events; the height of the _thick gray bars_denotes the release probability at the time of arrival of a spike. Parameter values: p0 = 0.9, N0= 8, τD = 2 sec. B, Trial-averaged release probability as a function of time. C, Steady-state synaptic response rate, given by the product of the average release probability and stimulation rate r. Because the average release probability behaves like 1/r, the response rate saturates at high stimulation frequencies. D, Histogram for the inter-release intervals in the steady state is close to an exponential with time constant τ = 〈IRI〉 = 1/(r〈pr〉ss) = 274 msec (solid line), where r = 20 Hz is the stimulation frequency, and 〈pr〉ss = 0.182 is the average steady-state release probability.
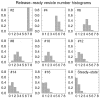
Fig. 3.
Evolution of the probability distribution P(N) for the number of available vesicles. In the top left corner is indicated the stimulus number in a 30 Hz train for which the histogram is computed. Synaptic parameters are the same as in Figure 2 (N0 = 8, τD = 2 sec, p0 = 0.9). Probability distribution is computed immediately before the spike. Initial distribution is a single peak at N = N0. The _last panel_shows the steady-state vesicle number distribution. In the steady state, the average number of available vesicles is typically one or two.
Fig. 4.
Temporal correlations of the release events in the steady state. A, Autocorrelation function of the release event sequence for p0 = 0.95 (αV = 0.374). B, Autocorrelation function for p0 = 0.6 (αV = 0.114). Parameters are N0 = 8, τD = 2 sec. Stimulation rate is r = 15 Hz. C, D, Autocorrelation function for the number of available vesicles. Parameters in C and D are the same as in A and B, respectively. Filled circles mark simulation results; solid curves are exponential fits with τcorr = 205 msec for_A_ and C, and τcorr = 535 msec for B and D.
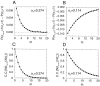
Fig. 5.
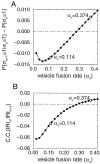
The sign of the steady-state temporal correlation depends on the vesicle fusion rate αV. _A,_Correlation between successive release events, as a function of αV. B, Coefficient of correlation between successive inter-release intervals as a function of αV. Both quantities become positive as αV is increased. Parameters are N0 = 8, τD = 2 sec, r = 15 Hz. Open circles mark points corresponding to αV values in Figure 4.
Fig. 6.
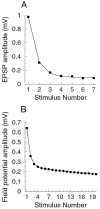
Examples of short-term depression at cortical synapses. A, Post-synaptic response to 20 Hz stimulation in rat layer 5 neocortical pyramidal neuron in slice, dual-cellular recording by Markram and Tsodyks (1996, their Fig. 2_B_). B, Amplitude of the field-potential response to 5 Hz stimulation of layer 4 recorded in layer 2/3 of rat visual cortex in slice. Figure was redrawn from Varela et al. (1997, their Fig. 4_C_).
Fig. 7.
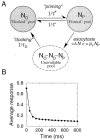
The two-step model of vesicle release.A, Model kinetics. Vesicles undergo “priming” before becoming available for release. Priming rate 1/τ+ is slower than the reverse rate 1/τ−, such that on average there is only one vesicle in the immediately releasable pool.B, Depression time course in response to a 30 Hz stimulation for the two-step synapse model. Parameter values are N0 = 6, τD = 2 sec, τ+ = 1.5 sec, τ− = 0.3 sec, αV = 4.6. Notice sharp depression of response after a single stimulus.
Fig. 8.
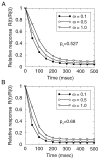
Behavior of the synapse model with unconstrained vesicle release. A, B, Response time courses for different values of the receptor saturation factor ω (between 0 and 1) specifying the degree to which postsynaptic receptors are saturated by neurotransmitter from a single vesicle. Response is measured by the average number of vesicles released and is normalized by initial response. Failure rate is 5% in A, and 1% in_B_. Other synaptic parameter are N0 = 4, τD = 2 sec. Stimulation rate is 15 Hz.
Fig. 9.
Temporal correlation of synaptic response is negative with unconstrained vesicle release. Correlation coefficient between the responses to two consecutive stimuli of a constant frequency stimulation train, as a function of the single-vesicle release probability, for two values of ω. Unlike in the case of the univesicular constraint (Fig. 5), here the correlation is always negative.
Fig. 10.
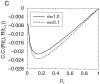
Effect of inhibitory metabotropic autoreceptors on synaptic transmission. Solid and _dashed lines_indicate model simulation data with and without presynaptic inhibition, respectively. A, Inter-pulse interval dependence of paired-pulse depression. Circles indicate experimental data obtained by Davies and Collingridge (1993) by recording inhibitory currents in pyramidal cells in rat hippocampal slices in control (filled circles) and in the presence of a GABAB antagonist (open circles). _B,Time course of depressing synaptic response to a 5 Hz stimulation.C, Steady-state release probability and (D) synaptic response rate (given by the product of release probability and the rate), as a function of the stimulation frequency. In contrast to Fig. 2_C, presynaptic inhibition prevents response rate saturation and extends the synaptic dynamic range. Synaptic parameter values are N0 = 6, p0 = 0.8, τD = 2 sec.
Fig. 11.
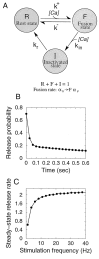
Inactivation of release machinery. _A,_Kinetic scheme for the model. A “gate” controlling vesicle release can be in one of the three states shown in the figure. With no stimulation, gates are predominantly in the R (rest) state. With stimulation, transition to the F (fusion) state takes place, leading to vesicle release. At the same time, quick transition from the F state to the inactive I state takes place, halting exocytosis. Internal Ca2+ is assumed to be an instantaneous function of presynaptic voltage, so Ca2+-driven transitions only occur during the brief time of stimulus arrival. B, Response time course for the release inactivation model with 30 Hz stimulation. Parameters are N0 = 6, τD = 2 sec, p0 = 0.7, k+ = 16.8 sec−1 μ
m
−1, k− = 333 sec−1, kin = 15 sec−1μ
m
−1, kr = 5 s−1, [Ca2+]pulse = 100 μ
m
, Δtpulse = 2 msec. Note the biphasic time course, the sharp paired-pulse depression followed by a slower deay process. C, Steady state response rate as a function of the stimulation frequency, reaching saturation at ∼20 Hz.
Similar articles
- Release Mode Dynamically Regulates the RRP Refilling Mechanism at Individual Hippocampal Synapses.
Kim Y, Lee U, Choi C, Chang S. Kim Y, et al. J Neurosci. 2020 Oct 28;40(44):8426-8437. doi: 10.1523/JNEUROSCI.3029-19.2020. Epub 2020 Sep 28. J Neurosci. 2020. PMID: 32989096 Free PMC article. - Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats.
Sun HY, Lyons SA, Dobrunz LE. Sun HY, et al. J Physiol. 2005 Nov 1;568(Pt 3):815-40. doi: 10.1113/jphysiol.2005.093948. Epub 2005 Aug 18. J Physiol. 2005. PMID: 16109728 Free PMC article. - Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus.
Dobrunz LE. Dobrunz LE. Int J Dev Neurosci. 2002 Jun-Aug;20(3-5):225-36. doi: 10.1016/s0736-5748(02)00015-1. Int J Dev Neurosci. 2002. PMID: 12175858 - Multiple vesicle recycling pathways in central synapses and their impact on neurotransmission.
Kavalali ET. Kavalali ET. J Physiol. 2007 Dec 15;585(Pt 3):669-79. doi: 10.1113/jphysiol.2007.137745. Epub 2007 Aug 9. J Physiol. 2007. PMID: 17690145 Free PMC article. Review. - Vesicle pools and short-term synaptic depression: lessons from a large synapse.
Schneggenburger R, Sakaba T, Neher E. Schneggenburger R, et al. Trends Neurosci. 2002 Apr;25(4):206-12. doi: 10.1016/s0166-2236(02)02139-2. Trends Neurosci. 2002. PMID: 11998689 Review.
Cited by
- Spatially structured oscillations in a two-dimensional excitatory neuronal network with synaptic depression.
Kilpatrick ZP, Bressloff PC. Kilpatrick ZP, et al. J Comput Neurosci. 2010 Apr;28(2):193-209. doi: 10.1007/s10827-009-0199-6. Epub 2009 Oct 29. J Comput Neurosci. 2010. PMID: 19866351 - GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus.
Mitchell SJ, Silver RA. Mitchell SJ, et al. J Neurosci. 2000 Dec 1;20(23):8651-8. doi: 10.1523/JNEUROSCI.20-23-08651.2000. J Neurosci. 2000. PMID: 11102470 Free PMC article. - Vesicle release probability and pre-primed pool at glutamatergic synapses in area CA1 of the rat neonatal hippocampus.
Hanse E, Gustafsson B. Hanse E, et al. J Physiol. 2001 Mar 1;531(Pt 2):481-93. doi: 10.1111/j.1469-7793.2001.0481i.x. J Physiol. 2001. PMID: 11230520 Free PMC article. - Short-term synaptic plasticity, simulation of nerve terminal dynamics, and the effects of protein kinase C activation in rat hippocampus.
Brager DH, Capogna M, Thompson SM. Brager DH, et al. J Physiol. 2002 Jun 1;541(Pt 2):545-59. doi: 10.1113/jphysiol.2001.015842. J Physiol. 2002. PMID: 12042358 Free PMC article. - Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum.
Chen BT, Rice ME. Chen BT, et al. J Neurosci. 2001 Oct 1;21(19):7841-7. doi: 10.1523/JNEUROSCI.21-19-07841.2001. J Neurosci. 2001. PMID: 11567075 Free PMC article.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources