Polarization of chemoattractant receptor signaling during neutrophil chemotaxis - PubMed (original) (raw)
Polarization of chemoattractant receptor signaling during neutrophil chemotaxis
G Servant et al. Science. 2000.
Abstract
Morphologic polarity is necessary for chemotaxis of mammalian cells. As a probe of intracellular signals responsible for this asymmetry, the pleckstrin homology domain of the AKT protein kinase (or protein kinase B), tagged with the green fluorescent protein (PHAKT-GFP), was expressed in neutrophils. Upon exposure of cells to chemoattractant, PHAKT-GFP is recruited selectively to membrane at the cell's leading edge, indicating an internal signaling gradient that is much steeper than that of the chemoattractant. Translocation of PHAKT-GFP is inhibited by toxin-B from Clostridium difficile, indicating that it requires activity of one or more Rho guanosine triphosphatases.
Figures
Fig. 1
Translocation of PHAKT-GFP to the plasma membrane of neutrophil-differentiated HL-60 cells. (A) PHAKT-GFP–expressing cells (I through III), C5aR-GFP–expressing cells (IV), and GFP-expressing cells (V) were differentiated to neutrophil-like cells (7) and plated on glass cover slips as described (4). Cells were stimulated either with a uniform increase in _f_MLP concentration, from 0 (I) to 100 nM (I′: 60 s post-stimulation) or with a point source of _f_MLP (1 μM) delivered by a Femtotip micropipette (10) (II′, III′, IV, and V), whose position is indicated by an asterisk. Panels II and III correspond, respectively, to cells shown in panels II′ and III′, but before stimulation with the micropipette. Images were recorded in the fluorescein isothiocyanate (FITC) channel every 5 s as described (4). Each result was reproduced in at least 10 experiments (11). Bars, 10 μm. Videos of a time course and asymmetric translocation of PHAKT-GFP are available at (9). (B) Extracellular gradient generated with the micropipette (I), relative to the intracellular gradient of PHAKT-GFP recruitment (II). For the experiment in (I), a Femtotips micropipette filled with sulforhodamine, a fluorescent dye, was lowered onto a cover slip overlaid with the buffer used for cell stimulation (4, 12); the image was taken in the rhodamine channel (4) 2 min after repositioning the micropipette in a field lacking dye fluorescence. The micropipette tip was located just outside the illumination field, at the left of the hexagon. The solid white line in (I) indicates the intensity of dye fluorescence ( _y_-axis) along the _x_-axis at the level of the black horizontal bar shown to the left of the figure; this curve presumably reflects the shape of a gradient of chemoattractant of similar molecular size, such as _f_MLP. The _x_-axis indicates distance across the microscopic field (solid white scale bar, 50 μm). Fluorescence intensities at the ends of this solid scale bar in (I) were 1900 and 300 (arbitrary units, after subtracting a background value of 400, obtained outside the hexagon). The dashed white curve in (I) indicates the intensity of PHAKT-GFP fluorescence measured across a diameter of the neutrophil shown in (II), depicted at the same scale as in (I). This cell was exposed to _f_MLP supplied by a micropipette; the diameter across which PHAKT-GFP fluorescence was measured is positioned at the level of the black horizontal bar in (II). The 15-μm dashed white line in (I) represents the intensity of PHAKT-GFP recruitment across this diameter; fluorescence intensities at the two ends of this line were 900 versus 200 (arbitrary units, after subtracting a background of 100 units measured outside the cell boundary). Maximum fluorescence intensity for rhodamine decreases to half its value over a distance of 30 μm, while maximum fluorescence intensity for PHAKT-GFP decreases to half its value over 5 μm.
Fig. 2
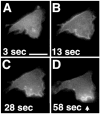
(A through D) Asymmetric translocation of PHAKT-GFP at the plasma membrane of neutrophil-differentiated HL-60 cells during polarization in response to a uniform increase in chemoattractant concentration. Differentiated cells (7) were plated on glass cover slips as described (4). Cells were stimulated with 100 nM _f_MLP at time 0 and images were then recorded as described in the legend of Fig. 1. The arrow in (D) points to the advancing leading edge of the cell. Uniform stimulation was assessed in 13 different sessions, recording the behavior of 68 cells (13). Bar, 10 μm. A video of this experiment is available at (9).
Fig. 3
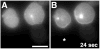
Asymmetric translocation of PHAKT-GFP in latrunculin-B–treated neutrophil-differentiated HL-60 cells. Differentiated cells (7) were plated on glass cover slips as described (4). Cells were then pretreated with 20 μg/ml latrunculin-B for 10 min (A) (14) and then stimulated, in the continued presence of the toxin, with a point source of _f_MLP (10 μM) delivered from a micropipette (10) [(B), asterisk]. Images were recorded as described in the legend of Fig. 1. Cells showed asymmetric recruitment biased by the micropipette’s position in five of nine stimulation sessions performed under these conditions. Bar, 10 μm.
Fig. 4
PHAKT-GFP translocation in cells treated with (A) PTX or C. difficile toxin-B and (B) a PI3K inhibitor, LY 294002 (14). (A) Neutrophil-differentiated cells (7), plated on glass cover slips (4), were stimulated sequentially with _f_MLP and insulin (panels II and III and panels V and VI, respectively) or C5a and insulin (panels VIII and IX, respectively). _f_MLP or C5a was added first, removed 2 min later, and replaced with insulin, then cells were stimulated for a further 3 min. Panel I through III: treatment with PTX (1 μg/ml). Panels IV through IX: treatment with C. difficile toxin-B (90 μg/ml). Panels I, IV, and VII: cells before stimulation with agonists (zero time). Images were recorded as described in the legend of Fig. 1. Stimulation times with the indicated agonists were as follows: II: 65 s; III: 115 s; V: 67 s; VI: 178 s; VIII: 39 s; IX: 161 s. Bars, 10 μm. (B) Effect of LY 294002 on chemoattractant- and insulin-induced plasma membrane translocation of PHAKT-GFP in neutrophil-differentiated HL-60 cells. Panels I, IV, and VII: untreated cells stimulated with insulin, _f_MLP, and C5a, respectively. Panels II, V, and VIII: unstimulated cells treated with LY 294002 (100 μM in panel II; 300 μM in panels V and VIII). Panels III, VI, and IX show responses of the same cells to insulin, _f_MLP, or C5a, respectively, in the presence of LY 294002 (100 μM in panel III; 300 μM in panels VI and IX). Images were recorded as described in the legend of Fig. 1. Stimulation times with agonists were as follows: I: 183 s; IV: 46 s; VII: 39 s; III: 199 s; VI: 61 s; IX: 41 s. For each treatment, the result shown is representative of at least eight different stimulation sessions performed on at least two different batches of neutrophil-differentiated HL-60 cells. Bars, 10 μm. Two additional panels of this figure (9) show the effect of 100 μM LY 294002 on chemoattractant-induced plasma membrane translocation of PHAKT-GFP (Web figure 4C) and a histogram (Web figure 4D) of percent cells responding to all three agonists tested in the presence of C. difficile toxin or LY 294002 at 100 or 300 μM.
Comment in
- Perspectives: signal transduction. Signals to move cells.
Dekker LV, Segal AW. Dekker LV, et al. Science. 2000 Feb 11;287(5455):982-3, 985. doi: 10.1126/science.287.5455.982. Science. 2000. PMID: 10691572 No abstract available.
Similar articles
- Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis.
Servant G, Weiner OD, Neptune ER, Sedat JW, Bourne HR. Servant G, et al. Mol Biol Cell. 1999 Apr;10(4):1163-78. doi: 10.1091/mbc.10.4.1163. Mol Biol Cell. 1999. PMID: 10198064 Free PMC article. - Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis.
Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Srinivasan S, et al. J Cell Biol. 2003 Feb 3;160(3):375-85. doi: 10.1083/jcb.200208179. Epub 2003 Jan 27. J Cell Biol. 2003. PMID: 12551955 Free PMC article. - Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils.
Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Xu J, et al. Cell. 2003 Jul 25;114(2):201-14. doi: 10.1016/s0092-8674(03)00555-5. Cell. 2003. PMID: 12887922 - Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products.
Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Rickert P, et al. Trends Cell Biol. 2000 Nov;10(11):466-73. doi: 10.1016/s0962-8924(00)01841-9. Trends Cell Biol. 2000. PMID: 11050418 Free PMC article. Review. - Signal transduction in neutrophil chemotaxis.
Katanaev VL. Katanaev VL. Biochemistry (Mosc). 2001 Apr;66(4):351-68. doi: 10.1023/a:1010293809553. Biochemistry (Mosc). 2001. PMID: 11403641 Review.
Cited by
- Spatiotemporal distribution of PTEN before directed cell migration in monolayers.
Lu Q, Sasaki S, Sera T, Kudo S. Lu Q, et al. In Vitro Cell Dev Biol Anim. 2024 Jun 26. doi: 10.1007/s11626-024-00927-x. Online ahead of print. In Vitro Cell Dev Biol Anim. 2024. PMID: 38926230 - Visualising Neutrophil Actin Dynamics in Zebrafish in Response to Laser Wounding Using Two-Photon Microscopy.
Williantarra I, Georgantzoglou A, Sarris M. Williantarra I, et al. Bio Protoc. 2024 Jun 5;14(11):e4997. doi: 10.21769/BioProtoc.4997. eCollection 2024 Jun 5. Bio Protoc. 2024. PMID: 38873016 Free PMC article. - Cell-Permeable Fluorescent Sensors Enable Rapid Live Cell Visualization of Plasma Membrane and Nuclear PIP3 Pools.
Kundu R, Kumar S, Chandra A, Datta A. Kundu R, et al. JACS Au. 2024 Mar 13;4(3):1004-1017. doi: 10.1021/jacsau.3c00738. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559732 Free PMC article. - Non-catalytic role of phosphoinositide 3-kinase in mesenchymal cell migration through non-canonical induction of p85β/AP2-mediated endocytosis.
Matsubayashi HT, Mountain J, Takahashi N, Deb Roy A, Yao T, Peterson AF, Saez Gonzalez C, Kawamata I, Inoue T. Matsubayashi HT, et al. Nat Commun. 2024 Mar 23;15(1):2612. doi: 10.1038/s41467-024-46855-y. Nat Commun. 2024. PMID: 38521786 Free PMC article. - Phosphoinositide Signaling in Immune Cell Migration.
Kakar R, Ghosh C, Sun Y. Kakar R, et al. Biomolecules. 2023 Nov 24;13(12):1705. doi: 10.3390/biom13121705. Biomolecules. 2023. PMID: 38136577 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous