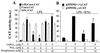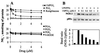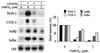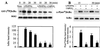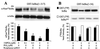Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-Delta(12,14)-prostaglandin J(2) in activated murine macrophages - PubMed (original) (raw)
Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-Delta(12,14)-prostaglandin J(2) in activated murine macrophages
A Castrillo et al. Mol Cell Biol. 2000 Mar.
Abstract
Activation of the macrophage cell line RAW 264.7 with lipopolysaccharide (LPS) and gamma interferon (IFN-gamma) induces the expression of gene products involved in host defense, among them type 2 nitric oxide synthase. Treatment of cells with 15-deoxy-Delta(12,14)-prostaglandin J(2) (15dPGJ(2)) inhibited the LPS- and IFN-gamma-dependent synthesis of NO, a process that was not antagonized by similar concentrations of prostaglandin J(2), prostaglandin E(2), or rosiglitazone, a peroxisomal proliferator-activated receptor gamma ligand. Incubation of activated macrophages with 15dPGJ(2) inhibited the degradation of IkappaBalpha and IkappaBbeta and increased their levels in the nuclei. NF-kappaB activity, as well as the transcription of NF-kappaB-dependent genes, such as those encoding type 2 nitric oxide synthase and cyclooxygenase 2, was impaired under these conditions. Analysis of the steps leading to IkappaB phosphorylation showed an inhibition of IkappaB kinase by 15dPGJ(2) in cells treated with LPS and IFN-gamma, resulting in an impaired phosphorylation of IkappaBalpha, at least in the serine 32 residue required for targeting and degradation of this protein. Incubation of partially purified activated IkappaB kinase with 2 microM 15dPGJ(2) reduced by 83% the phosphorylation in serine 32 of IkappaBalpha, suggesting that this prostaglandin exerts direct inhibitory effects on the activity of the IkappaB kinase complex. These results show rapid actions of 15dPGJ(2), independent of peroxisomal proliferator receptor gamma activation, in macrophages challenged with low doses of LPS and IFN-gamma.
Figures
FIG. 1
NF-κB binding is inhibited in activated RAW 264.7 cells treated with 15dPGJ2. (A) Macrophages were incubated for 1 h with different combinations of 15dPGJ2 (2 μM), PGJ2 (2 μM), rosiglitazone (10 μM), LPS (500 ng/ml), and IFN-γ (10 U/ml). After homogenization of the cells, nuclear extracts were prepared and the binding of nuclear proteins to the distal κB motif of the NOS-2 promoter was determined by EMSA. Supershift assays with Abs against proteins of the c-Rel family (not shown) identified p50-p50 and p50-p65 as the complexes present in the lower and upper bands, respectively. (B) Dose-dependent effect of 15dPGJ2 on NF-κB activity. The binding of nuclear proteins to the PPARα R response element of the acyl-CoA oxidase promoter was used as control of extraction of nuclear proteins and lane load. The intensity of the bands was determined, and the corresponding values are expressed as mean ± SEM of three experiments (A). ∗, P < 0.005 with respect to the LPS/IFN-γ condition. a.u., arbitrary units.
FIG. 2
Subcellular distribution of IκBα and IκBβ in cells treated with 15dPGJ2. Macrophages were incubated for 45 min with LPS (500 ng/ml) and 15dPGJ2 (1 μM). After the cells were fixed, IκB proteins were detected with specific Abs and revealed using Cy3-labeled anti-rabbit Ig. (A) The intensity of the fluorescence in the cytosolic and nuclear compartments was digitalized and quantified (n = 14 to 21 cells per condition). (B) The corresponding amount of IκBα and IκBβ present in cytosolic and nuclear extracts was determined also by Western blotting. Results show the mean ± SEM of three experiments. ∗, P < 0.05 with respect to the corresponding LPS condition.
FIG. 3
15dPGJ2 inhibits CAT expression in cells transfected with (κB)3ConA.CAT and p2iNOS.CAT vectors. Macrophages were transfected with DOTAP, and cells were incubated with the indicated stimuli followed by activation with LPS (500 ng/ml) (A) or LPS (500 ng/ml) plus IFN-γ (10 U/ml) (B). After 14 h of incubation with the indicated stimuli, CAT activity was determined. Transfection with ConA.CAT and kSV2.CAT was used to ensure that the ligands did not affect the basal activity of the promoters and the efficiency of the transfection. Results show the mean ± SEM of three experiments. ∗, P < 0.01 with respect to the LPS or LPS/IFN-γ condition.
FIG. 4
Inhibition of NOS-2 expression by 15dPGJ2 in RAW 264.7 cells activated with LPS/IFN-γ. Cells were treated for 5 min with the indicated concentrations of PGs, rosiglitazone and hydroxyoctadecadienoic acid (9-HODE), followed by activation with LPS (200 ng/ml) and IFN-γ (10 U/ml). (A) After 18 h of incubation,the amount of nitrite and nitrate in the culture medium was measured. (B) The dose-dependent effect of 15dPGJ2 on NOS-2 levels was determined by Western blotting, using the levels of the phosphatidylinositol 3-kinase subunit p85α as a control of lane charge. Results show the mean of three experiments (A) and a representative blot out of two (B).
FIG. 5
15dPGJ2 decreases the mRNA levels of COX-2 and NOS-2. Cells were incubated with the indicated concentrations of PG and activated with LPS (200 ng/ml) and IFN-γ (10 U/ml). After 4 h of treatment, the cells were homogenized and the RNA was extracted and analyzed by Northern blotting using probes specific for the indicated genes. Results show the mean band intensity expressed as a percentage of that in the absence of PG and after normalization for the content of 18S rRNA (n = 3).
FIG. 6
Inhibition of IκBα phosphorylation in activated RAW 264.7 cells treated with 15dPGJ2. Macrophages were incubated with 2 μM 15dPGJ2 5 min prior to stimulation with LPS (200 ng/ml) and IFN-γ (10 U/ml). At the indicated times, cell extracts were prepared, IKK was immunoprecipitated, and the in vitro kinase activity of 100 ng of IP protein was assayed using GST-IκBα(1-317) and [γ-32P]ATP as substrates. (A) After 10 min of incubation, GST-IκBα was purified with glutathione-agarose and analyzed by SDS-PAGE (10% gel). Aliquots (5 μl) of the kinase reaction mixture were analyzed by Western blotting to determine the amount of IKK2 present in each assay. (B) Cells treated with 10 μM MG132 were stimulated as described previously; at the indicated times, cytosolic extracts were prepared and the amount of endogenous P(Ser32)IκBα was determined using a specific Ab. The blot was reprobed with anti-IκBα Ab. The intensity of the bands of phosphorylated IκBα (A) and the ratio between the band intensities of P(Ser32)IκBα and IκBα (B) are given. Results show the mean ± SEM of three experiments. ∗, P < 0.005 with respect to the corresponding LPS/IFN-γ condition.
FIG. 7
Inhibition of IKK activity by 15dPGJ2. Macrophages were incubated for 20 min with LPS (200 ng/ml) and IFN-γ (10 U/ml); after homogenization, the IKK complex was immunoprecipitated with anti-IKK2 Ab. Kinase activity was monitored in vitro for 10 min, using GST-IκBα(1-317) as the substrate and in the presence of the indicated concentrations of PGs and rosiglitazone. P(Ser32)IκBα was detected using a specific anti-P(Ser32)IκBα Ab. The membrane was reprobed with anti-IκBα Ab (A). The incorporation of [32P]phosphate into GST-IκBα(1-54) or IκBαS/A was determined as previously described (B). Results show the mean ± SEM of the band intensities, expressed with respect to the condition in the absence of PG and rosiglitazone. ∗, P < 0.01 with respect to the condition in the absence of addition.
Similar articles
- Potentiation of protein kinase C zeta activity by 15-deoxy-delta(12,14)-prostaglandin J(2) induces an imbalance between mitogen-activated protein kinases and NF-kappa B that promotes apoptosis in macrophages.
Castrillo A, Través PG, Martín-Sanz P, Parkinson S, Parker PJ, Boscá L. Castrillo A, et al. Mol Cell Biol. 2003 Feb;23(4):1196-208. doi: 10.1128/MCB.23.4.1196-1208.2003. Mol Cell Biol. 2003. PMID: 12556480 Free PMC article. - Regulation and function of IKK and IKK-related kinases.
Häcker H, Karin M. Häcker H, et al. Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
- Cacospongionolide B suppresses the expression of inflammatory enzymes and tumour necrosis factor-alpha by inhibiting nuclear factor-kappa B activation.
Posadas I, De Rosa S, Terencio MC, Payá M, Alcaraz MJ. Posadas I, et al. Br J Pharmacol. 2003 Apr;138(8):1571-9. doi: 10.1038/sj.bjp.0705189. Br J Pharmacol. 2003. PMID: 12721113 Free PMC article. - An endoplasmic reticulum stress-initiated sphingolipid metabolite, ceramide-1-phosphate, regulates epithelial innate immunity by stimulating β-defensin production.
Kim YI, Park K, Kim JY, Seo HS, Shin KO, Lee YM, Holleran WM, Elias PM, Uchida Y. Kim YI, et al. Mol Cell Biol. 2014 Dec;34(24):4368-78. doi: 10.1128/MCB.00599-14. Epub 2014 Oct 13. Mol Cell Biol. 2014. PMID: 25312644 Free PMC article. - Insulin resistance: Is it time for primary prevention?
Mercurio V, Carlomagno G, Fazio V, Fazio S. Mercurio V, et al. World J Cardiol. 2012 Jan 26;4(1):1-7. doi: 10.4330/wjc.v4.i1.1. World J Cardiol. 2012. PMID: 22279598 Free PMC article. - 15-Deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2) mediates repression of TNF-alpha by decreasing levels of acetylated histone H3 and H4 at its promoter.
Engdahl R, Monroy MA, Daly JM. Engdahl R, et al. Biochem Biophys Res Commun. 2007 Jul 20;359(1):88-93. doi: 10.1016/j.bbrc.2007.05.057. Epub 2007 May 21. Biochem Biophys Res Commun. 2007. PMID: 17532302 Free PMC article. - Peroxisome proliferator activated receptor gamma: a potential therapeutic target in the management of ischaemic heart disease.
Sidhu JS, Kaski JC. Sidhu JS, et al. Heart. 2001 Sep;86(3):255-8. doi: 10.1136/heart.86.3.255. Heart. 2001. PMID: 11514473 Free PMC article. Review. No abstract available.
References
- Chinetti G, Griglio S, Antonucci M, Torra I P, Delerive P, Majd Z, Fruchart J C, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. - PubMed
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
- Colville-Nash P R, Qureshi S S, Willis D, Willoughby D A. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J Immunol. 1998;161:978–984. - PubMed
- D'Acquisto F, Sautebin L, Iuvone T, Di Rosa M, Carnuccio R. Prostaglandins prevent inducible nitric oxide synthase protein expression by inhibiting nuclear factor-κB activation in J774 macrophages. FEBS Lett. 1998;440:76–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials