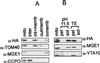A DNA helicase required for maintenance of the functional mitochondrial genome in Saccharomyces cerevisiae - PubMed (original) (raw)
A DNA helicase required for maintenance of the functional mitochondrial genome in Saccharomyces cerevisiae
T Sedman et al. Mol Cell Biol. 2000 Mar.
Abstract
A novel DNA helicase, a homolog of several prokaryotic helicases, including Escherichia coli Rep and UvrD proteins, is encoded by the Saccharomyces cerevisiae nuclear genome open reading frame YOL095c on the chromosome XV. Our data demonstrate that the helicase is localized in the yeast mitochondria and is loosely associated with the mitochondrial inner membrane during biochemical fractionation. The sequence of the C-terminal end of the 80-kDa helicase protein is similar to a typical N-terminal mitochondrial targeting signal; deletions and point mutations in this region abolish transport of the protein into mitochondria. The C-terminal signal sequence of the helicase targets a heterologous carrier protein into mitochondria in vivo. The purified recombinant protein can unwind duplex DNA molecules in an ATP-dependent manner. The helicase is required for the maintenance of the functional ([rho(+)]) mitochondrial genome on both fermentable and nonfermentable carbon sources. However, the helicase is not essential for the maintenance of several defective ([rho(-)]) mitochondrial genomes. We also demonstrate that the helicase is not required for transcription in mitochondria.
Figures
FIG. 1
Schematic representation of the S. cerevisiae YOL095c gene and loss of mitochondrial respiratory functions in ΔYOL095c strains. (A) YOL095c ORF. The black boxes indicate the positions of the seven helicase motifs. The strategy used to construct disruption strain via replacement of the internal _Bgl_II-_Eco_RI fragment by the TRP1 marker is illustrated. The amino acid sequences of the seven helicase motifs are compared to the corresponding consensus sequences. +, hydrophobic residue; O, polar residue; X, any residue in the consensus structure. (B) Analysis of the phenotypic effect of the YOL095c ORF disruption. The heterozygous strain TS103 was sporulated, and tetrads were analyzed on glucose-containing SCM plates (left panel) or glycerol-containing SCG plates (right panel). (C) Growth of four haploid colonies originating from one tetrad, analyzed on different media. All four haploids are viable on glucose-containing SCM plates (right); two haploids have the YOL095c ORF disrupted with the TRP1 gene, as indicated by growth on the −TRP plate (left). The haploids with disrupted YOL095c ORF fail to grow on the glycerol plate (middle).
FIG. 2
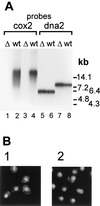
Loss of functional mtDNA in Δ_hmi1_ strains. (A) Southern blot of total cellular DNA. DNA, isolated from the wt strain W303-1A (wt) and from the haploid Δ_hmi1_ colonies (Δ), was cut with _Eco_RI (lanes 1, 2, 5, and 6) or _Bam_HI (lanes 3, 4, 7, and 8) and probed with mitochondrial COX2 (lanes 1 to 4) or nuclear DNA2 (lanes 5 to 8) probes. (B) DNA staining in nuclei and mitochondria of the wt W303-1A strain (panel 1) and of a haploid cell population from a colony where the HMI1 ORF has been disrupted (panel 2).
FIG. 3
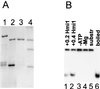
The recombinant Hmi1 protein is a DNA helicase. (A) Purification of the Hmi1 protein. Lane 1, glutathione agarose eluate; lane 2, thrombin-cleaved material; lane 3, 400 mM S-Sepharose fraction; lane 4, protein size markers BSA (68 kDa), ovalbumin (45 kDa), and trypsinogen (24 kDa). (B) DNA unwinding assay with the recombinant Hmi1 protein. Lane 1, assay with 0.2 μg of Hmi1p; lanes 2 to 4, assays with 0.4 μg of Hmi1; lane 3, omitted ATP; lane 4, omitted MgCl2; lane 5, substrate DNA; lane 6, heat-denatured substrate.
FIG. 4
Active transcription in mitochondria of Δ_hmi1_ strains. Total cellular RNAs from [_rho_0] strain SK088 and [_rho_−] hypersuppressive strains SK061 and SK041 were analyzed by dot blot hybridization. The amount of total RNA loaded per dot is indicated. Lanes marked “+KOH” represent alkali-treated samples. Lanes marked “+HMI1” are samples from strains that were transformed with the HMI1 complementing plasmid pRS315-HMI1. The blot was probed with a mitochondrial ori2 probe (left panel) and then stripped and probed with a cytoplasmic 25S rRNA probe (right panel).
FIG. 5
Biochemical fractionation localizes the Hmi1 protein in mitochondria. (A) Analysis of intracellular localization of the Hmi1 protein. Yeast cells (strain W303-1A), transformed with the expression construct YCAH-HMI1, and mitochondria were isolated by using fractionation by differential centrifugation. Mitochondria were subfractionated further, and the fractions were analyzed by Western blotting. The blots were probed with monoclonal anti-HA antibody 12CA5 (HA) or rabbit polyclonal antibodies against TOM40 (outer membrane protein), CCPO (intermembrane space protein), or MGE1 (peripheral inner membrane protein). Lane 1, mitochondria; lane 2, intermembrane space; lane 3, matrix plus membranes; lane 4, outer membrane; lane 5, inner membrane. (B) The Hmi1 protein is not an integral inner membrane component. Mitochondria were diluted, sonicated briefly, and extracted on ice with 0.1 M sodium carbonate (pH 11.5) (lanes 2 and 3) or with 10 mM Tris (pH 8.0)–1 mM EDTA (lanes 4 and 5). Soluble and membrane-bound fractions were separated by centrifugation for 1.5 h at 35,000 rpm in the Beckman Ti70 rotor. Pellet (pel) and supernatant (sol) fractions were analyzed by Western blotting with anti-HA monoclonal antibody 12CA5 and polyclonal antibodies against YTA10p (integral inner membrane protein) or Mge1p (peripheral inner membrane protein).
FIG. 6
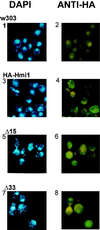
Colocalization of mtDNA and the Hmi1 protein or the Hmi1 mutants in situ. Yeast cells expressing the tagged Hmi1 protein or the C-terminal deletion mutants were fixed, and indirect immunostaining was used to analyze the intracellular localization of the Hmi1 protein or its mutants (right panels). mtDNA was stained in the same samples by using DAPI (left panels). Panels 1 and 2, W303-1A; panels 3 and 4, W303-1A transformed with YCAH-HMI1; panels 5 and 6, W303-1A transformed with YCAH-ΔC15Ala; panels 7 and 8, W303-1A transformed with YCAH-ΔC33Gly.
FIG. 7
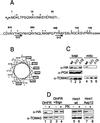
The C-terminal segment of the Hmi1 helicase is required for correct targeting of the protein into mitochondria. (A) Sequences of the N- and C-terminal segments of the Hmi1 protein. The acidic (−) and basic (+) amino acid residues are indicated above the sequence. The endpoints of the two C-terminal deletion mutants Mrh-ΔC15Ala and Mrh-ΔC33Gly are indicated with arrows below the C-terminal sequence segment. (B) Helical wheel presentation of the 18 C-terminal amino acid residues starting from Arg-691. Hydrophobic residues are indicated with boxes, and the positively charged residues are indicated with a “+.” (C) Western blot analysis of whole-cell extracts and mitochondrial fractions from the W303-1A strain transformants expressing the Hmi1 protein or the corresponding deletion mutant. Lanes 1 and 4, W303-1A transformed with YCAH-HMI1; lanes 2 and 5, W303-1A transformed with YCAH-ΔC33Gly; lanes 3 and 6, W303-1A transformed with YCAH-ΔC15Ala. The blots were probed with the anti-HA antibody 12CA5, anti-TOM40 (mitochondrial marker), and anti-PGK (cytoplasmic marker). (D) The C-terminal segment of the Hmi1 protein targets the DHFR carrier protein into mitochondria, and introduction of negatively charged residues in the C-terminus of the Hmi1 protein abolishes the mitochondrial import. The following proteins were expressed in W303-1A strain cells: lanes 1 and 2, HA-tagged DHFR; lanes 3 and 4, the fusion protein of HA-DHFR and the C-terminal segment (residues 616 to 706); lanes 5 and 6, HA-tagged Hmi1 protein; and lanes 7 and 8, mutant HA-Hmi1-Asp12. A Western blot shows analysis of total cellular protein (lanes 1, 3, 5, and 7) and the mitochondrial fraction treated with proteinase K for 10 min on ice (lanes 2, 4, 6, and 8). The blots were probed with anti-HA antibody 12CA5 and anti-TOM40.
Similar articles
- Helicase Hmi1 stimulates the synthesis of concatemeric mitochondrial DNA molecules in yeast Saccharomyces cerevisiae.
Sedman T, Jõers P, Kuusk S, Sedman J. Sedman T, et al. Curr Genet. 2005 Apr;47(4):213-22. doi: 10.1007/s00294-005-0566-4. Epub 2005 Feb 3. Curr Genet. 2005. PMID: 15690159 - Biochemical and genetic characterization of Hmi1p, a yeast DNA helicase involved in the maintenance of mitochondrial DNA.
Monroe DS Jr, Leitzel AK, Klein HL, Matson SW. Monroe DS Jr, et al. Yeast. 2005 Dec;22(16):1269-86. doi: 10.1002/yea.1313. Yeast. 2005. PMID: 16358299 - Mitochondrial helicase Irc3 translocates along double-stranded DNA.
Sedman T, Garber N, Gaidutšik I, Sillamaa S, Paats J, Piljukov VJ, Sedman J. Sedman T, et al. FEBS Lett. 2017 Dec;591(23):3831-3841. doi: 10.1002/1873-3468.12903. Epub 2017 Nov 22. FEBS Lett. 2017. PMID: 29113022 - Yeast and human mitochondrial helicases.
Szczesny RJ, Wojcik MA, Borowski LS, Szewczyk MJ, Skrok MM, Golik P, Stepien PP. Szczesny RJ, et al. Biochim Biophys Acta. 2013 Aug;1829(8):842-53. doi: 10.1016/j.bbagrm.2013.02.009. Epub 2013 Feb 27. Biochim Biophys Acta. 2013. PMID: 23454114 Review. - [Structure and diversity of yeast mitochondrial genomes].
Okamoto K, Sekito T, Kitano H, Yoshida K. Okamoto K, et al. Tanpakushitsu Kakusan Koso. 1994 Aug;39(10):1638-50. Tanpakushitsu Kakusan Koso. 1994. PMID: 8090935 Review. Japanese. No abstract available.
Cited by
- Schizosaccharomyces pombe pfh1+ encodes an essential 5' to 3' DNA helicase that is a member of the PIF1 subfamily of DNA helicases.
Zhou JQ, Qi H, Schulz VP, Mateyak MK, Monson EK, Zakian VA. Zhou JQ, et al. Mol Biol Cell. 2002 Jun;13(6):2180-91. doi: 10.1091/mbc.02-02-0021. Mol Biol Cell. 2002. PMID: 12058079 Free PMC article. - Replication intermediates of the linear mitochondrial DNA of Candida parapsilosis suggest a common recombination based mechanism for yeast mitochondria.
Gerhold JM, Sedman T, Visacka K, Slezakova J, Tomaska L, Nosek J, Sedman J. Gerhold JM, et al. J Biol Chem. 2014 Aug 15;289(33):22659-22670. doi: 10.1074/jbc.M114.552828. Epub 2014 Jun 20. J Biol Chem. 2014. PMID: 24951592 Free PMC article. - Search for protein partners of mitochondrial single-stranded DNA-binding protein Rim1p using a yeast two-hybrid system.
Kucejová B, Foury F. Kucejová B, et al. Folia Microbiol (Praha). 2003;48(2):183-8. doi: 10.1007/BF02930953. Folia Microbiol (Praha). 2003. PMID: 12807077 - Yeast exonuclease 5 is essential for mitochondrial genome maintenance.
Burgers PM, Stith CM, Yoder BL, Sparks JL. Burgers PM, et al. Mol Cell Biol. 2010 Mar;30(6):1457-66. doi: 10.1128/MCB.01321-09. Epub 2010 Jan 19. Mol Cell Biol. 2010. PMID: 20086101 Free PMC article.
References
- Alexandre C, Grueneberg D A, Gilman M Z. Studying heterologous transcription factors in yeast. Methods Companion Methods Enzymol. 1993;5:147–155.
- Daum G, Böhni P C, Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space. J Biol Chem. 1982;257:13028–13033. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases