Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes - PubMed (original) (raw)
Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes
C Y Loo et al. J Bacteriol. 2000 Mar.
Abstract
Viridans streptococci, which include Streptococcus gordonii, are pioneer oral bacteria that initiate dental plaque formation. Sessile bacteria in a biofilm exhibit a mode of growth that is distinct from that of planktonic bacteria. Biofilm formation of S. gordonii Challis was characterized using an in vitro biofilm formation assay on polystyrene surfaces. The same assay was used as a nonbiased method to screen isogenic mutants generated by Tn916 transposon mutagenesis for defective biofilm formation. Biofilms formed optimally when bacteria were grown in a minimal medium under anaerobic conditions. Biofilm formation was affected by changes in pH, osmolarity, and carbohydrate content of the growth media. Eighteen biofilm-defective mutants of S. gordonii Challis were identified based on Southern hybridization with a Tn916-based probe and DNA sequences of the Tn916-flanking regions. Molecular analyses of these mutants showed that some of the genes required for biofilm formation are involved in signal transduction, peptidoglycan biosynthesis, and adhesion. These characteristics are associated with quorum sensing, osmoadaptation, and adhesion functions in oral streptococci. Only nine of the biofilm-defective mutants had defects in genes of known function, suggesting that novel aspects of bacterial physiology may play a part in biofilm formation. Further identification and characterization of biofilm-associated genes will provide insight into the molecular mechanisms of biofilm formation of oral streptococci.
Figures
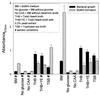
FIG. 1
Bacterial growth and biofilm formation of S. gordonii Challis under different growth conditions. Bacteria were grown in either BM, BM without glucose, BM without CAA, THB, THB+YE, or TSB. Growth and biofilm formation were measured under aerobic (#) and anaerobic conditions. All assays were performed in triplicate, and mean values and standard deviations are shown.
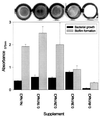
FIG. 2
Bacterial growth and biofilm formation of S. gordonii Challis in BM with different levels of osmolarity. The NaCl supplement in BM varied from 0 to 0.4 M. Assays were performed using BM and polystyrene plates under anaerobic conditions. A representative row of CV-stained microtiter plate wells is shown above the graph.
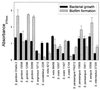
FIG. 3
Bacterial growth and biofilm formation of various oral streptococci. Assays were performed using BM and polystyrene plates under anaerobic conditions.
FIG. 4
SEM micrographs of S. gordonii Challis biofilm formation on uncoated and coated polystyrene surfaces. (A) Uncoated polystyrene; (B) laminin; (C) type IV collagen; (D) fibronectin. Magnification is shown by the bar (10 μm). Two different magnifications are shown for each surface (×1,000 for the upper images and ×3,000 for the lower images).
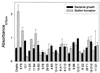
FIG. 5
Bacterial growth and biofilm formation of wild-type S. gordonii Challis and biofilm-defective mutants. Assays were performed using BM and polystyrene plates under anaerobic conditions.
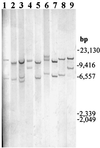
FIG. 6
Southern hybridization of Hin_dIII-digested chromosomal DNAs from nine representative biofilm-defective mutants with a DIG-labeled pAM120 probe containing Tn_916. Lanes: 1, 8F9 mutant; 2, 1C1 mutant; 3, 11E5 mutant; 4, 11B4 mutant; 5, 15B3 mutant; 6, 9F8 mutant; 7, 29E5 mutant; 8, 4B3 mutant; 9, 13A12 mutant. DNA sizes are shown on the right.
Similar articles
- Insertional inactivation of genes responsible for the D-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations.
Clemans DL, Kolenbrander PE, Debabov DV, Zhang Q, Lunsford RD, Sakone H, Whittaker CJ, Heaton MP, Neuhaus FC. Clemans DL, et al. Infect Immun. 1999 May;67(5):2464-74. doi: 10.1128/IAI.67.5.2464-2474.1999. Infect Immun. 1999. PMID: 10225909 Free PMC article. - Involvement of the adc operon and manganese homeostasis in Streptococcus gordonii biofilm formation.
Loo CY, Mitrakul K, Voss IB, Hughes CV, Ganeshkumar N. Loo CY, et al. J Bacteriol. 2003 May;185(9):2887-900. doi: 10.1128/JB.185.9.2887-2900.2003. J Bacteriol. 2003. PMID: 12700268 Free PMC article. - Identification of a novel two-component system in Streptococcus gordonii V288 involved in biofilm formation.
Zhang Y, Lei Y, Khammanivong A, Herzberg MC. Zhang Y, et al. Infect Immun. 2004 Jun;72(6):3489-94. doi: 10.1128/IAI.72.6.3489-3494.2004. Infect Immun. 2004. PMID: 15155656 Free PMC article. - Involvement of an inducible fructose phosphotransferase operon in Streptococcus gordonii biofilm formation.
Loo CY, Mitrakul K, Voss IB, Hughes CV, Ganeshkumar N. Loo CY, et al. J Bacteriol. 2003 Nov;185(21):6241-54. doi: 10.1128/JB.185.21.6241-6254.2003. J Bacteriol. 2003. PMID: 14563858 Free PMC article. - Quorum sensing in streptococcal biofilm formation.
Suntharalingam P, Cvitkovitch DG. Suntharalingam P, et al. Trends Microbiol. 2005 Jan;13(1):3-6. doi: 10.1016/j.tim.2004.11.009. Trends Microbiol. 2005. PMID: 15639624 Review.
Cited by
- An In Vitro Model for Oral Mixed Biofilms of Candida albicans and Streptococcus gordonii in Synthetic Saliva.
Montelongo-Jauregui D, Srinivasan A, Ramasubramanian AK, Lopez-Ribot JL. Montelongo-Jauregui D, et al. Front Microbiol. 2016 May 12;7:686. doi: 10.3389/fmicb.2016.00686. eCollection 2016. Front Microbiol. 2016. PMID: 27242712 Free PMC article. - Streptococcus mutans copper chaperone, CopZ, is critical for biofilm formation and competitiveness.
Garcia SS, Du Q, Wu H. Garcia SS, et al. Mol Oral Microbiol. 2016 Dec;31(6):515-525. doi: 10.1111/omi.12150. Epub 2016 Feb 4. Mol Oral Microbiol. 2016. PMID: 27753272 Free PMC article. - Subpopulation behaviors in lactose metabolism by Streptococcus mutans.
Zeng L, Burne RA. Zeng L, et al. Mol Microbiol. 2021 Jan;115(1):58-69. doi: 10.1111/mmi.14596. Epub 2020 Oct 6. Mol Microbiol. 2021. PMID: 32881164 Free PMC article. - Experimental Models of Oral Biofilms Developed on Inert Substrates: A Review of the Literature.
Darrene LN, Cecile B. Darrene LN, et al. Biomed Res Int. 2016;2016:7461047. doi: 10.1155/2016/7461047. Epub 2016 Sep 6. Biomed Res Int. 2016. PMID: 27699173 Free PMC article. Review. - Mutation of luxS affects biofilm formation in Streptococcus mutans.
Merritt J, Qi F, Goodman SD, Anderson MH, Shi W. Merritt J, et al. Infect Immun. 2003 Apr;71(4):1972-9. doi: 10.1128/IAI.71.4.1972-1979.2003. Infect Immun. 2003. PMID: 12654815 Free PMC article.
References
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases