Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo - PubMed (original) (raw)
Figure 1
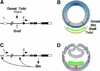
Regulation of sim transcription along the DV axis. (A) Genomic map of the sim gene showing positions of the early (E) and late (L) promoters and intron–exon structure. Open boxes correspond to untranslated regions, and closed boxes to coding sequence. At stage 5, transcriptional activation of the E promoter is positively regulated by Dorsal, Twist, and Notch signaling, and inhibited by Snail. (B) Schematic cross section of the Drosophila blastoderm embryo after cellularization (stage 5) (adapted from Campos-Ortega and Hartenstein 1997). The DV gradient of nuclear localization of Dorsal is shown in blue. Dorsal is mostly nuclear in ventral cells, and predominantly cytoplasmic in dorsal cells. In ventral nuclei, peak levels of Dorsal activate transcription of the mesoderm-determining genes twist and snail, and repress transcription of dorsal fate-determining genes. The domains of snail and twist expression are shown in green and yellow, respectively. In lateral nuclei, lower levels of Dorsal activate the transcription of neuroectoderm-determining genes, such as short gastrulation and rhomboid, which are repressed ventrally by Snail. Separating the ventral neuroectoderm from the mesoderm is the mesectoderm, a single row of cells on either side of the embryo. Mesectodermal cells, which express sim, are in red. In dorsal nuclei, levels of Dorsal are too low to repress the expression of genes determining dorsal fates. (C) Sim autoregulates its own transcription from both early (E) and Late (L) promoters from stage 8 onward. (D) Schematic cross section of a gastrulating embryo (stage 8). Adapted from Campos-Ortega and Hartenstein (1997). Following invagination of the mesoderm (in green), mesectodermal cells (in red) merge at the midline, and, after two rounds of cell division, differentiate into 22–26 midline neurons and glia per segment (Klambt et al. 1991).
Figure 2
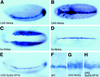
Regulation of sim expression by Notch signaling. In situ hybridization of wild-type (A_–_C) and Notch mutant (D_–_F) embryos showing the distribution of sim transcripts at stages 5 (A,D), 6 (B,E), and 8 (C,F). (A,B) Expression of sim was detected in a single row of mesectodermal cells in wild-type embryos [ventrolateral (A) and ventral (B) views]. Accumulation of sim transcripts at the posterior pole is out of focus. (C) Expression of sim in midline cells in a wild-type stage 8 embryo (ventral view). (D,E) In Notch mutant embryos, the expression of sim was restricted to a few cells in the mesectoderm. High levels of sim transcripts were seen at the posterior pole [ventrolateral (D) and ventral (E) views]. (F) The expression of sim was detected in very few midline cells in Notch mutant embryos at stage 8 (ventral view). Two null mutant alleles of Notch, N55e11 (D_–_F) and N5419 (not shown), were used in this study and gave similar results. In A_–_F anterior is to the left.
Figure 3
Ectopic expression of sim in the ventral neuroectoderm. In situ hybridization of Matα4–GAL–VP16/UAS–Nintra (A,B,G), hs-Nintra (C,D), Matα4–GAL–VP16/UAS–Su(H)–VP16 (E,H), and wild-type (F) embryos showing the distribution of sim transcripts. (A) Lateral view showing the ectopic expression of sim in the neuroectoderm of a stage 5 Matα4–GAL–VP16/UAS–Nintra embryo. Expression of sim was not detected in the mesoderm. Mesodermal cells were identified here as the cells that invaginate into the ventral furrow; in Matα4–GAL–VP16/UAS–Nintra embryos, all of the cells located between the two bands of _sim_-expressing cells invaginate to form the mesoderm. (B) Ventral view of a stage 10 embryo showing that this ectopic expression of sim was seen persisting after mesoderm invagination. (C) Transient overexpression of hs-Nintra at stage 5 induced the ectopic expression of sim in the neuroectoderm (ventral view). No sim expression was detected in the mesoderm. (D) Ventral view of a stage 8 hs-Nintra embryo showing that heat-induced expression of Nintra did not affect sim expression after mesoderm invagination. (E) Lateral view of a stage 5 Matα4–GAL–VP16/UAS–Su(H)–VP16 embryo. Nintra and Su(H)–VP16 similarly induced the ectopic expression of sim in the neuroectoderm. (F_–_H) Higher magnification views of the embryos shown in Figs. 2A (F; wild-type control), 3A (G; UAS–Nintra), and 3E [H; UAS–Su(H)–VP16].
Figure 4
Molecular and phenotypical analysis of Su(H)del47. (A) Schematic representation of the l(2)35Bg_–_Su(H) genomic region. The four Su(H) exons appear as boxes. The ORF of Su(H) is shown in black. The positions of the Su(H) and l(2)35Bg transcriptional starts are indicated by arrows. Numbering refers to the transcriptional start of Su(H) (+1). The genomic structure of the l(2)35Bg gene has not been determined. The 4.2-kb _Eco_RI–_Eco_RI DNA fragment used as a probe for Southern blot analysis (B) is shown as a black line (_Eco_RI). The MR1 allele of Su(H) results from the insertion at position +65 of a defective P element. Its imprecise excision generated a 1.9-kb deletion called del47. Sequence analysis of a PCR product encompassing the deletion breakpoint showed that it actually corresponds to the substitution of a 1881-bp DNA fragment by 9 unrelated nucleotides. Su(H)del47 did not complement Su(H) and l(2)35Bg lethal alleles. In addition, a P element containing a 6.8-kb genomic DNA fragment encoding the transcription unit called B in Schweisguth and Posakony (1992), P[l(2)35Bg+], rescues the embryonic lethality associated with Su(H)del47 and l(2)35Bg mutant alleles (data not shown; the 6.8-kb DNA fragment used for genomic rescue is shown as a hatched bar). These results demonstrate that l(2)35Bg corresponds to the B transcription unit located 5′ to Su(H) and deleted in Su(H)del47. (B) Genomic Southern blot hybridization analysis of the del47 allele. _Eco_RI-digested genomic DNA was analyzed with the 4.2-kb _Eco_RI fragment shown in A as a hybridization probe. The 5.4-, 4.2-, and 2.3-kb bands correspond to the MR1, wild-type, and del47 alleles of Su(H), respectively. (C,D) Cuticular preparations of Su(H)SF8 (C) and Su(H)del47 P[l(2)35Bg+] (D) mutant embryos. The phenotype of Su(H)del47 appears to be slightly stronger than the one associated with Su(H)SF8, but is significantly milder than the one resulting from a loss of Notch activity (Zecchini et al. 1999).
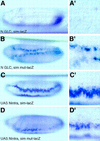
Figure 5
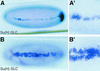
Reduced and ectopic expression of sim in Su(H) mutant embryos. In situ hybridization of Su(H)del47 P[l(2)35Bg+] mutant embryos showing the distribution of sim transcripts at stages 5 (A,A′) and 8 (B,B′). (A,A′) Low levels of sim expression were detected in two to three cell rows at stage 5 (lateral view; A′ is an enlarged view of the same embryo). Accumulation of sim transcripts at the posterior pole is out of focus. (B,B′) Occasionally, a few cells located at the midline failed to express sim, creating small gaps. In other regions, cells expressing high levels of sim transcripts were observed in one to two rows of cells away from the midline at stage 8, forming small clusters (ventral views). No discernable pattern of gaps and clusters was recognized.
Figure 6
Identification of Su(H)-binding sites in the sim regulatory region. (A) Alignment of the predicted Su(H)-binding sites (Su1 to Su10) contained within a 2.8-kb upstream regulatory region of sim to the consensus Su(H)-binding site. The core consensus is shown in black uppercase letters; the two residues flanking the core consensus are less conserved. Sites Su4, Su5, Su7, Su8, Su9, and Su10 perfectly match the core consensus. Sites Su1, Su2, Su3, and Su6 differ at one conserved position (as indicated in red). Putative site c differs at a position shown previously to be essential for CBF-1/RBP-Jk binding (Tun et al. 1994). The sim regulatory sequence contains no other sites differing by less than two conserved nucleotides. Two binding sites from the Enhancer of split m8 gene [_E(spl)-m8_] were used as positive controls (Bailey and Posakony 1995; Lecourtois and Schweisguth 1995). For each putative site, the relative binding affinity, as estimated from gel shift assays, is indicated on the right. (+++, ++, +, +/−) Very high, high, medium, and weak binding affinity, respectively; (−) no detectable binding. Site c, which differs from the consensus at a strictly conserved position, did not bind Su(H). (B) Schematic diagrams of the upstream regions of the sim genes from D. virilis and D. melanogaster (Kasai et al. 1998). The position of the predicted Su(H)-binding sites is shown relative to the predicted Snail-, Twist-, and Dorsal-binding sites. The conserved regions that include known binding sites are underlined. These correspond to regions 2a, 3, 5, 10, 14, 15, and 16 described in Kasai et al. (1998). Four binding sites predicted to bind strongly Su(H) (Su9, Su8, Su7, and Su5) appeared to be clustered with predicted Snail-, Twist-, and Dorsal-binding sites in both D. virilis and D. melanogaster. Nucleotide numbering refers to the translation initiation codon. (C) Gel retardation analysis of Su(H) binding to putative sites from the sim regulatory region. Radiolabeled 17-mer oligonucleotides centered around putative Su(H) binding sites were tested for their ability to form retarded complex with Su(H) in an EMSA. One site perfectly matching the core consensus, Su7, as well as all the sites differing by one nucleotide to the core consensus (Su6, Su3, Su2, Su1, and c) were analyzed. For each probe, free lysate was used as a negative control (lanes 1,3,5,7,9,11,13). In vitro translated Su(H) proteins bound strongly to Su7 (lane 2). Weak binding was also observed with Su6, Su3, Su2, and Su1 (lanes 6,8,10,12, respectively). No detectable binding was observed with putative site c (lane 14). Binding specificity was demonstrated with an oligonucleotide containing two mutations in the Su7 site, Su7m (lane 4). These results are consistent with the binding specificity displayed by the mouse homolog of Su(H) (Tun et al. 1994), as the Su7m and c sites are the only ones that contain nucleotides differing from the consensus at strictly conserved position. (D) Determination of relative binding affinities by competition EMSA. Increasing amounts (5×, 10×, and 20×) of nonlabeled oligonucleotides were tested for their ability to compete with the formation of radiolabeled Su7–Su(H) complex (lanes 1_–_3). The m8a (lanes 10_–_12) and Su7 (lanes 4_–_6) oligonucleotides efficiently competed the binding of Su(H) to Su7. The m8b (lanes 13_–_15), Su6 (lanes 16_–_18), Su3 (lanes 19_–_21), Su2 (lanes 22_–_24), and Su1 (lanes 25_–_27) oligonucleotides competed only weakly. The Su7m (lanes 7_–_9) and c (lanes 28_–_30) oligonucleotides did not show significant competition activity. The plot underneath the EMSA gel shows the quantitation of the radioactivity contained within retarded complexes as measured by PhosphorImager analysis. The radioactivity measured in the absence of specific competitor was chosen as the 100% reference (lane 3).
Figure 7
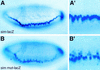
The Su(H)-binding sites are required to repress sim transcription in the neuroectoderm. Lateral views of wild-type sim–lacZ (A,A′) or simmut–lacZ (B,B′) embryos showing the distribution of lacZ transcripts at stage 6. lacZ transcripts accumulated in a single row of mesectodermal cells in sim–lacZ embryos (A,A′). In contrast, they were detected in several rows of cells extending into the ventral neuroectoderm in simmut–lacZ embryos (B,B′; cf. A′ and B′). (A′,B′) Enlarged views of the embryos shown in A and B. These embryos are homozygous and express two copies of the transgene (A,A′,B,B′).
Figure 8
Su(H) acts via the Su(H)-binding sites to repress sim transcription. Lateral views of wild-type embryos (A,A′,B,B′) and ventrolateral views of Su(H)del47 P[l(2)35Bg+] mutant embryos (C,C′,D,D′) and Matα4–GAL–VP16/UAS–Su(H)–VP16 embryos (E,E′,F,F′) showing the expression pattern of sim–lacZ (A,A′,C,C′,E,E′) and simmut–lacZ (B,B′,D,D′,F,F′) transgenes at stage 6. All embryos have only one copy of the same transgene. A reduced level of staining was observed in embryos carrying one copy of the sim–lacZ or simmut–lacZ transgenes (A_–_B′) compared with embryos homozygous for the same transgenes (Fig. 7_A_–B′). For both sim–lacZ (C,C′) and simmut–lacZ (D,D′), low levels of lacZ expression were detected in two to three cell rows in Su(H) mutant embryos. Expression of Su(H)–VP16 resulted in the ectopic expression of sim–lacZ in the neuroectoderm (E,E′). In contrast, expression of simmut–lacZ did not appear to be significantly up-regulated by Su(H)–VP16 (cf. F,F′ with B,B′).
Figure 9
The repression mediated by the Su(H)-binding sites does not require Notch activity. In situ hybridization of N55e11 mutant embryos derived from GLC (A,A′,B,B′; ventral views) and Matα4–GAL–VP16/UAS–Nintra embryos (C,C′,D,D′; ventrolateral views) showing the expression pattern of sim–lacZ (A,A′,C,C′) and simmut–lacZ (B,B′,D,D′) transgenes at stage 6. Loss of Notch activity abolished sim–lacZ transcription (A,A′), but did not affect the expression of simmut–lacZ (cf. B,B′ with Fig. 7_B,B′_). Expression of activated Notch resulted in the ectopic expression of sim–lacZ in the neuroectoderm (C,C′). In contrast, expression of simmut–lacZ was not significantly modified by the expression of Nintra (cf. D,D′ with Fig. 7_B,B′_).
Figure 10
A model for the transcriptional activation of the sim gene in a single row of cells. Cross-section of a blastoderm embryo at stage 5. As in Fig. 1, the DV gradient of nuclear localization of Dorsal is shown in blue, the mesectoderm is in red. The sharp border of Snail accumulation (in green) coincides with the mesoderm–mesectoderm boundary. Accumulation of Twist (in yellow) gradually fades away in the neuroectoderm. In the neuroectoderm (1), transcriptional activation by low levels of Dorsal and Twist is inhibited by the Su(H)-mediated repression. In the mesectoderm (2), Notch activation relieves the repression mediated by Su(H) and, together with Dorsal and Twist, stimulates the expression of sim. In the mesoderm (3), Snail represses sim transcription, and overcomes the positive regulation mediated by Dorsal and Twist. Whether Su(H) and Notch participate in regulating sim in the mesoderm is unknown.